Document Type : Review Article
Authors
1 Physics Department, College of Science, University of Halabja, 46018, Halabja, Iraq
2 Department of Physics, Faculty of Science, Firat University, 23119, Elazig, Turkey
Abstract
A researcher needs to know all the chemical, physical, biological, structural, and mechanical characteristics of a biomedical material before using it in medical applications. The mineral hydroxyapatite (HAp, Ca10(PO4)6(OH)2) is an essential component of the calcium orthophosphate family. It possesses great dielectric and biological compatibility properties, diamagnetic properties, thermal stability, osteoconductivity, and bioactivity, which has a Ca:P molar ratio of 1.67. Since HAp has a chemical makeup that is very similar to that of natural bone and teeth, it has the potential to be utilized as a material for the implantation of implants in broken parts of the human skeletal system. Because of the increasing use of HAp in medicine, many methods for producing HAp nanoparticles have been discovered. The preparation conditions of synthesized HAp determine their physical and chemical characteristics, crystalline structure, and shape. This study gives a comprehensive information on the properties and production methods of the HAp in detail and unveile the structure and its properties in detail.
Graphical Abstract
Keywords
Main Subjects
- Introduction
More materials, dubbed biomaterials, have been progressively highlighted in biological fields during the previous four decades. However, the concept of "biomaterials" in materials science and clinical medicine may have been interpreted differently. A biomaterial is a synthetic material implanted to replace live tissue to restore normal physiological functions. This type of synthetic substance has some bioactivity. Biomaterials are divided into two categories: (1) Natural (collagen, elastin, and gelation) and (2) artificial (metals, alloys, ceramics, composites, and polymers) [1].
Researchers are researching the relationships between materials structures, features, preparation techniques, and application functions in material science, which is an interdisciplinary field concerned with recognizing and using the qualities of materials. It comprises various materials, with biomaterials being one of the most important and useful [2]. Bioceramics materials are prepared in many dissimilar phases to obtain the required properties and function. They can be single crystals (sapphire), polycrystalline glass (bio glass), glass-ceramics (apatite/wollastonite, A/W), or composites (polyethylene/HAp, HAp/Alumina) [3]. Calcium orthophosphate-based bioceramics are now available. The literature reports on the industrial and agricultural qualifications of its most important ceramic biomaterial samples, focusing on calcium orthophosphates. HAp and tricalcium phosphate (TCP), with the formula of Ca3(PO4)2 found in two different forms of α and b phases, are the most popular members of the calcium orthophosphates [4].
HAp is almost comparable to the inorganic component of bones and teeth and has several essential characteristics. Due to its great biocompatibility, thermal stability, high bioactivity, and lack of toxicity, it is frequently used in biomedical applications. Because of its poor mechanical characteristics, hydroxyapatite's use in orthopedics is limited. Aside from that, hydroxyapatite may swap its ions with various foreign ions, potentially improving biocompatibility, mechanical properties, and microstructure [5-6].
Researchers are turning to implant materials for this reason and have already utilized them to mend and replace damaged skeletal tissues. Calcium orthophosphates are a kind of bioceramic that has been widely employed in medicine, particularly in the field of tough tissue engineering. More recently, bioceramics research and development have made important contributions to human health and quality of life. Because it makes up a large percentage of the basic structure of tooth and bone minerals, HAp, is a major biomaterials utilized in this sector, has gained much attention in medical applications. Ca10(PO4)6(OH)2 is the chemical formula for HAp, which has a Ca:P molar ratio of 1.67. HAp appears to be a helpful material appropriate for hard tissue recover procedures with high biocompatibility, osteoconductivity ability, and bioactivity characteristics, resulting in no human body rejection [7]. One of the most frequent supporting materials that has been utilized with a variety of semiconductors, including TiO2, Ag3PO4, and AgBr/Ag3PO4, is called hydroxyapatite (HAp). According to certain reports, HAp can both enhance the surface area of catalytic material and adsorb pollutant particles, resulting in faster breakdown of the pollutants [8].
HAp is rarely used in a few load-bearing orthopedic applications due to its poor mechanical properties. Sinterability, morphology, grain size, and phase composition are all elements that influence mechanical characteristics [9,10]. The main objective of these process are to generate HAp powders with desired properties, such as large surface areas, fine grain size, and shape, including low particle aggregation [11]. Every one of these features of HAp powders was dependent on the technique used. The wet-chemical reaction, in particular, is the most talented way due to its ease of procedure, low working temperature, high percentages of pure products, and low equipment required [12,13]. To characterize the HAp, the following approaches were utilized: (a) After dissolving in a dilute HC1 solution, a chemical analysis was performed; P as determined by the colorimetric method of Fogg and Wilkinson [14], Ca be measured using atomic emission spectrophotometry. (b) XRD analysis determines the degree of crystallinity. (c) The continuous flow method of Nelsen and Eggertsen is used to create surface area. (d) Magnifications of upwards of 50,000 in microscopy of the electron. (e) Solubility measurements were made above a pH range of 6-7 by adding H3PO4 to apatite solutions and equilibration for 20 days before calculating P and Ca in suspension [15,16].
The preparation conditions of synthesized HAp determine their physical and chemical characteristics, crystalline structure, and shape. Calcium phosphate compounds of varying compositions, like amorphous calcium phosphates (ACPs), are frequent phases in synthetic apatites generated by precipitating in super-saturation aqueous solutions [16]. Two major types of synthetic HAp are monoclinic and hexagonal [17]. Generally, the most common factors influencing HAp during preparation include temperature, amount of pH, kind of element, shape of crystals, crystallite size, and preparation method. This present study aimed to show a property, structure, medical application, and numerous methods of HAp’s fabrication, and compare preparation techniques.
- Literature
This section covered a range of important topics and outlined some theoretical basis notions that would be useful in later chapters and understanding research findings. It starts with a basic explanation of biomaterials, and then discusses biomaterial usage in bone building and the dental complex. Finally, bioceramic types and biocompatibility are discussed.
2.1. HAp as a biomaterial
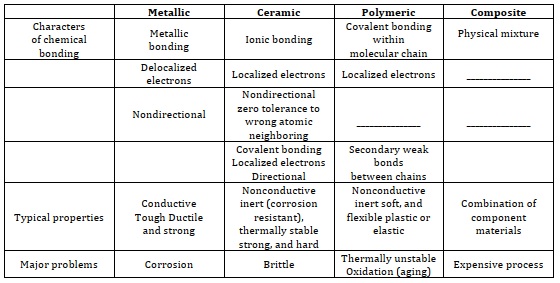
Biomaterials are materials that are meant to be utilized on their own or as components of more complex medical devices to bring infected areas or broken tissue into contact with the biological system. Biomaterials can be either synthetic or natural. [18]. Table 1 indicates four materials types biomaterials research, as is typical of the 21st century science, necessitates significant expertise in medical sector, materials science, biochemistry, bio-medical engineering, and clinical sector, as well as multidisciplinary collaboration from numerous key disciplines. Any man-made or organic compounds that come into direct touch with live cells or body fluids and the vital system in the human body are referred to as biomedical materials. These materials include joints and commercial metal plates utilized to mend broken bones, and they are required to attain full bio-compatibility with the body to be considered safe [19, 6].
Table 1. Four materials types and their major characteristics [7]
However, because biomaterials research focuses on biological responses and biochemical reactions of synthetic materials, the study of the property-structure connection, which is a popular issue in engineering materials, may focus more on bio-activity behaviors than bio-engineering characteristics. However, because the primary uses of bio-materials are in medical applications, biomedical sciences have become an essential element of biomaterials research. Some examples of this include cells and genetics, molecular anatomy, and both human and animal physiology. During an implant, the interface between the replacement bio-material and the biological tissue is a critical issue that must be investigated [18].
The fact that both of these materials, as well as other biomaterials that have been used in the past, are biocompatible is the fundamental distinction between them. Biocompatibility refers to the fact that biomaterials can be managed by themselves and do not cause harm to the living tissue or its surroundings. Biomaterials have a favorable influence because they have the potential to treat a wide variety of medical conditions affecting many different bodily systems [1, 20]. In addition, the enhancement of in vitro testing, one of the medical uses of biomaterials employed in cancer research, is an essential component of the biological applications of cancer treatments. In vitro systems provide a more accurate description of pharmacological endeavors and drug-delivery techniques. The exploration of biomedical applications of biological material has resulted in a great deal of research and experimental information that is extremely helpful to once again heal both soft and hard matter into body. It is depicted in Figure 1. One of the most typical instances of a ceramic biomaterial, HAp is a biomaterial used extensively in recovering bones and teeth. In this chapter, the structure and components of bone and teeth will be discussed in more detail [4]. When an implant made of synthetic product is placed inside of a human body, the surrounding tissue responds to the implant in different ways, depending on the type of material utilized. In general, we can classify three main types of biomaterials.
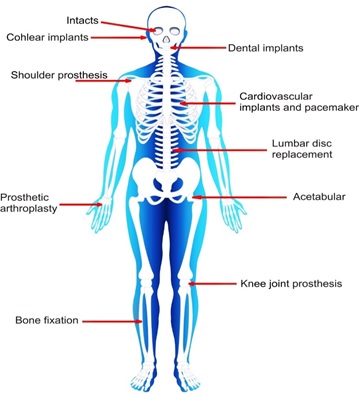
Figure 1. Various parts of the body are subject to medical that include the use of biomaterials [4]
- Bioceramics
In a variety of uses in medicine, bioceramics are utilized as solid or transparent substances with specific morphologies, such as implants, replacements, and prosthetic devices [21, 11] that show the schematic of structure, characteristics, and processing.
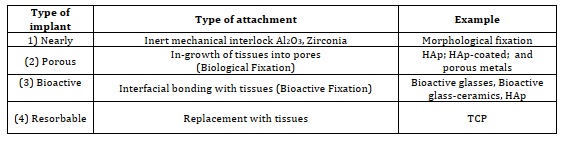
Due to their outstanding biocompatibility, bio-ceramics, which are specific ceramic products designed for medical and dental reasons, have been regarded as the most preferable materials for mending hard tissues. HAp is extremely comparable to the inorganic portion of human hard tissues like bones and teeth [11-13, 21-23]. The field of bioceramics can be separated into four distinct groups, as presented in Table 2.
Table 2. Types of tissue attachment of bioceramic prostheses [24]
3.1. Types of bioceramics
Due to structure, composite, chemical composition, crystal structure, and properties, bioceramics can be classified into three types (inert bioceramics, bioactive bioceramics, and biodegradable bioceramics).
3.1.1. Inert bioceramics
When placed in a living organism, no substance remains unharmed. When placed in tissue that lives, the best material will not suffer any significant chemical modifications and will have ano effect. Materials that are bio-inert keep their chemical, electrical, and mechanical characteristics for an extended period of time in the host tissue [14]. The thickness of fibrous tissue is evaluated in molecular layers because bio-inert ceramics like aluminum oxide (Al2O3) and zirconium oxide (ZrO2) have a greater degree of resistance to biological growth than composites.
3.1.2. Bioactive bioceramics
Bioactivity is defined as the capacity to create an adhesive contact with tissues. Bioactive ceramics refers to bio-active glasses, glass ceramics, and HAp as a whole [15, 25]. It is possible to categorize the most prevalent bio-active materials according to their bioactivities, and reactivity processes [16, 26].
According to the Bioactivity Index (IB), bioactive substances can be divided into two distinct categories, as detailed in the following table:
- Class A: materials with an IB value > 8, actively interact with tissues and induce both soft and hard tissue intrinsic repair and regenerative bonding, such as the bioactive glass and glass-ceramic.
Note/ IB that relates to the amount of time it takes for 50% of the interface to be bonded: IB = 100/t0.5bb.
Here, (t0.5bb) is the quantity of time it takes for more than 50% of the interface to attach to bone.
- Class B: materials with IB < 8, but greater than 0 are considered osteoconductivity materials only, e.g., synthetic HAp and beta-tri calcium phosphate (β-TCP) [27].
For over 20 years, there has been some success with calcium phosphate-based Ca3(PO4)2 bioceramics being employed in medical practices. Because of the same bone apatite, hydroxyapatite has played an important role in the calcification and resorption of bone [28]. When placed in touch with water-based solutions while at room temperature, there are only two Ca3(PO4)2 compounds that are stable, and the pH of the resulting solution will determine which of the two can remain stable:
- At a pH lower than 4.2 pH, the component CaHPO4.2H2O (dicalcium phosphate) is the most stable.
- While the stable phase is HAp at high temperatures (until 1,000 °C) for calcium phosphates when pH > 4.2. Other phases, such as Ca3(PO4)2 (TCP) and Ca4P2O9 (TTCP) may become visible at higher temperatures in the region between 1,000 °C and 1,500 °C [18, 29]. Error! Reference source not found. Figure 2 depicts the examples of stable and unstable anion-cation pairs [10, 30].
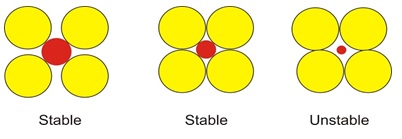
Figure 2. Stable and unstable anion-cation is (positively) coordination configurations. Yellow circles represent anions (Negative); red circles denote cations [30]
3.1.3. Biodegradable bioceramics
The development of biodegradable orthopedic implants has evolved substantially during the previous decade [31-32]. In recent years, there has been a lot of focus placed on biodegradable biomaterials. This is due to the fact that the ideal materials for bone repair should have a biosorption rate that is proportional to the pace at which new bone is formed. The well-known biodegradable materials are dicalcium phosphate (DCP, CaHPO4), calcium carbonate (CaCO3), calcium sulfate (CaSO4), PLLA (poly-lactide), etc. [33-34]. Calcium phosphate, denoted as Ca:P coatings, are applied to the contact between a bone and an implant to stimulate new bone development [35]. Ca/P-based coatings, such as HAp and tricalcium phosphate (TCP), are recognized for their high bioactivity and, as a result, their potential to stimulate bone regeneration [36]. Because of its comparable shape and composition to the mineral phase of bone, HAp has been widely recognized as a viable option for covering metallic implants [37].
The degree to which calcium phosphates degrade is influenced both by the pH of the medium in which they are found and the solubility of the substance itself. It is different for any structure in the following order (HAp << β-TCP< α-TCP). The degree to which Ca3(PO4)2 have degraded is proportional to the crystals grain size, the crystallinity level, the size of the grains, and the surface area of the calcium phosphate crystals [24, 35, 38].
- Hydroxyapatite
Hydroxyapatite (HAp) is a kind of ceramic that belongs to the apatite family. The name "apatite" comes from the Greek word apatite, which means "deception." It was given this name because of its variety in form and color [39-42]. Apatite is the term given to a group of compounds, that have a molecular density in the range of 3.16×10-3 to 3.14×10-3 g/mm3 and have the formula Y10(ZO4)6X2. In the formula Y10(ZO4)6X2, Y denotes a divalent metal cation (Ca2+), Z signifies a pentavalent phosphorus cation (P5+), and X represents an anionic radical, specifically one of Cl-, F-, or OH- [43-48]. Because it has structure similar to genuine human bone, it may be utilized in the body to replace damaged parts of the human skeleton system, such as medication administration, the development of bones promoter, tooth and bone cement, bone fillers, tooth canal filler, and dental implants. dental implants are also used to replace missing teeth [49]. Biomedical applications of several biomaterials are being identified to improve human health. Calcium orthophosphates restore teeth and bones in animals. Nonorganic biomaterials like calcium orthophosphates may help tissues stay rigid and stable [50-52]. The term "bioceramic" refers to a relatively recent technological development that has resulted in the creation of nanoparticles with physical, biochemical, and biomechanical properties that make them appropriate for orthopedic and dental implantation [44]. Whole calcium orthophosphates exist in the form of orthophosphate anions and have three original constituents. These compositions are phosphorus (P5+), calcium (Ca2+), and oxygen (O2-). The Ca/P molar ratio of whole calcium orthophosphates ranges between 2 and 0.5 [53]. Al2O3, TiO2, and ZrO2 are common ceramics used in the human body [54].
HAp Ca10(PO4)6(OH)2 is a calcium phosphate that in morphology and composition is identical to the human hard tissues. The calcium phosphates with the most significance in the human body are HAp, which has a Ca: P molar ratio of 1.67, and TCP, which has a Ca: P molar ratio of 1.5 [49]. The ideal Ca/P molar ratio of HAp is 10/6, and the calculated density is 3.219 g/mL [55]. The majority of a mammal's skeletal structure, including their teeth and bones, is composed of (HAp, Ca10(PO4)6(OH)2), a substance that is bioactive, biologically compatible, and osto-conductive. HAp is also known as apatite. Due to the fact that it possesses exceptional biological qualities, it has also found usage in a variety of medical treatments. [56-59]. Synthetic HAp materials have stronger crystallinity and structural stability than natural bone apatite, which is detrimental to bone development and the breakdown of synthetic materials [60]. The addition of these trace elements found in real bone into synthetic hydroxyapatite can lower the crystallinity and structural stability of HAp while also improving degradation and biological performance [61-62].
Researchers are turning to implant materials for this reason, and have already utilized them to recover and replace human tissues. Calcium orthophosphates are a kind of bioceramic that has been widely employed in medicine, particularly in the field of hard tissue engineering. More recently, bioceramics research and development have made important contributions to human better life. HAp has seen a great deal of application in the area of biomedicine because it has a high level of bioactivity, biocompatibility, non-toxicity, and stability. Between entire calcium orthophosphates, HAp is the most stable in thermochemical various environmental factors such as pH, temperature, and content of the human biological conditions. The HAp bioactivity is one of its characteristics, and it has the potential to induce bone development and regeneration. The amount of time needed to repair damage caused by HAp is much less than the amount of time needed to repair damage caused by other macro-size biomaterials of a similar sort due to HAp's superior bioactivity and improved resorbability at a temperature of 1200 degrees Celsius, the HAp phase was generated; however, beyond that temperature, it decomposed into another phase known as TCP [63, 64]. To be able to create HAp, metal components on Ca/P may be obtained in a range of phases, depending on the technique, with several features, such as crystallographic defects, surface area, organic compounds, grain size, and so on. These metal components can be obtained in any of these phases using any of these methods [63].
Multiple methods exist for synthesizing HAp. The wet method is popular because it is simple to implement, can be performed at room temperature, provides a high concentration of a pure product, poses minimal risk, and requires nothing in the way of costly apparatus. The wet method involves adding a solvent to water, in which biochemical reactions among calcium and phosphorus ions take place at a pH that is carefully regulated and at an appropriate temperature [63,65-66]. The stable phase is [DCPD (CaHPO4•2H2O)] at 4.2 > pH, and the stable phase HAp at 4.2 < pH [67]. Table 3 lists the phases of calcium phosphate, which are dependent on the Ca:P molar ratio. The ceramics HAp and TCP are the most often utilized in the medical field [68]. Synthetic HAp materials have stronger crystallinity and structural stability than natural bone apatite, which is detrimental to bone development and the breakdown of synthetic materials [60]. The addition of these trace elements found in real bone into synthetic hydroxyapatite can lower the crystallinity and structural stability of HAp while also improving degradation and biological performance [61, 62].
Table 3. Compounds of calcium phosphate that are the most significant overall [69]
The molar ratio of Ca/P is directly responsible for the high density and good mechanical properties of ceramic HAp. If the Ca:P < 1.67, (α,β-TCP) can be investigated. The HAp is stable at a 1.67 Ca: P molar ratio and calcium oxide (CaO) occurs after sintering if the HAp Ca:P atomic ratio is greater than 1.67. The existence of CaO is shown to reduce strength [70].
By shifting from an anionic substitution kind to a cationic replacement type, calcium may be substituted by ions such as OH- and PO43-. HAp is characterized by many features, one of which is poor bone-binding. For example, to enhance or develop the physical behavior, bonding, electrical properties, biological activity, and mechanical characteristics of HAp and other ceramics, a range of anions and cations may be doped or co-doped by different additions anionic such as. Examples of these types of additions include (CO32-, SiO44-, Cl-, and F-, etc.), and additions cationic like (Mn2+, Zn2+, Li+, Ce3+, Na+, K+, Ag+, Sr2+, and Mg2+, and so on). Because it is only used in a few loads in medical applications, pure HAp exhibits poor mechanical behavior when compared to the other components of the human body. The Ca2+ radius has connections to the mechanical behavior, which can be influenced by the size of the particles and form, lattice constants, surface morphology, solubility, physicochemical, and biological reaction of the component [3, 24, 67, 71-74]. To improve antibacterial and bioactivity in biomedical areas such as therapy, tomography, and diagnostics, for instance, Cerium (Ce) is one of the rare earth elements (REE) that is used extensively. This is due to the amazing magnetic, optical, and catalytic properties that these elements possess [4].
4.1 Apatite
One source of apatite is biological, while the other is inorganic deposits, such as phosphate rock or phosphorite, a sedimentary rock in which carbonate fluorapatite is the essential nutrients component. Apatite can be found in both biological and inorganic forms [39]. See Figure 3 for the apatite formula unit illustration. Apatite is a phosphate mineral group which uses a general formula for this group. The four most frequent types of apatite are as follows:
- Hydroxy-apatite — Ca10(PO4)6(OH)2
- Chlor-apatite — Ca10(PO4)6Cl2
- Fluor-apatite — Ca10(PO4)6F2
- Brom-apatite — Ca10(PO4)6Br2
Figure 3. The hydroxyapatite formula unit looks like [75]
4.2. General formula of hydroxyapatite
The general formula of these minerals could be M10(PO4)6X2.
where,
- M might be any one of a number of different metals (usually Calcium, Ca),
- P usually stands for shorthand for phosphorous, and
- X is commonly a hydroxide (OH) or a halogen such as chlorine (Cl-) or fluorine (F-).
The term "apatite" is currently used to refer to a family of compounds that have similar structural characteristics (hexagonal system, space group, and P63/m) despite having a broad variety of chemical make-ups, as indicated in Table 4 [43, 76].
Table 4. The formula of apatite [7]

4.3. Structure of hydroxyapatites
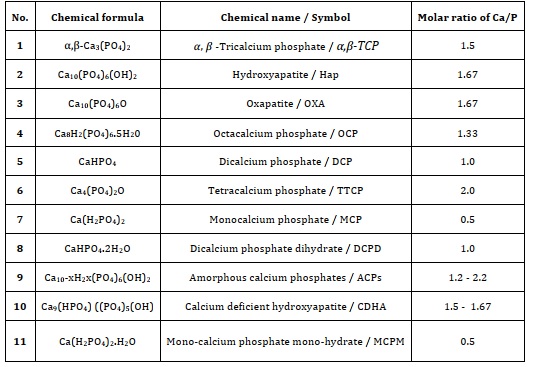
One of the bioactive ceramics that has received the greatest research attention is hydroxyapatite. HAp is composed of the same ions as are found in the metallic component of teeth and bones. Therefore, it shares the same chemical properties as the mineral that is found in bone and other types of hard tissue in humans. This gives HAp the ability to perform the same functions as the mineral [77]. HAp Structure has a single-phase and hexagonal system unit cell crystal structure, where a = b ≠ c, α = β= 90°, and γ = 60°. The system belongs to the spacing group P63/m and the approximate lattice parameters a = b = 0.9418 nm and c = 0.6884 nm [4, 78-81].
Ca2+, PO43- and OH- ions are pure stoichiometric HAp. Figure 4 illustrates a hexagonal construction. Each unit cell in the 1 lattice contains one atom of Ca2+ located in one of the corners, and one atom of OH- is located in the middle of the unit cell [82].
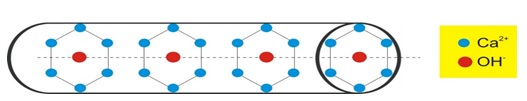
Figure 4. Hexagonal lattice component of apatite [1]
As demonstrated in Figure 5, Pure HAp has a chemical structure that consists of the ions Ca2+, PO43-, and OH- among other elements. In conclusion, a single unit cell of pure HAp has the components 2OH, 10Ca, and 6P. May be divided into two separate groups: Ca (I) and Ca (II). Ca (I) places are located in the centre of the hexagonal array, and occupied by four calcium atoms, whereas Ca (II) locations, which are located in the corners of the hexagonal column and surrounds OH- ions, are inhabited by six calcium atoms [1, 48, 54].
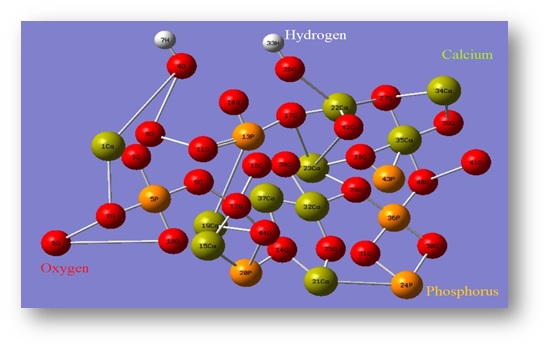
Figure 5. Crystal structure of pure HAp
4.3.1. Bone structure
The components that makeup bones are both inorganic and organic, which gives them their rigidity. The quality and strength of the bone (mass bone), which includes blood and cells, is essential. The ability of silicate-glass elements to bind with bone was originally noted by Larry Hench in 1969. Silicate-glass components would always have strong chemical stability (biocompatibility), as well as the potential to bond with the bone [24, 83-85]. Ceramic biological materials made from calcium and phosphorus, including HAp and b-TCP, have always been demonstrated beyond a reasonable doubt to aid in the restoration of bone [83]. The basic structure of the bone is comprised of three primary significant properties: (i) matrix of bone, (ii) collagen, and (iii) hydroxycarbonate apatite. HAp is a naturally occurring substance that shares the same ionic makeup as bone, teeth, and other types of hard tissue found within the body. To start understanding the chemical makeup of HAp, one must comprehend the fundamental components of tissues.
The major components of natural bone tissue are calcium phosphate (69 wt %), water (10 wt %), collagen (20 wt %), and other organic elements in tiny quantities, like proteins, polysaccharides, and fats, as illustrated in Figure 6 [77]. A rise in calcium Ca2+ is often excellent for the quality of bone health and the prevention of osteoporosis. It is also beneficial to the development of bone, the defense of bone, and the breaking of bone [4].
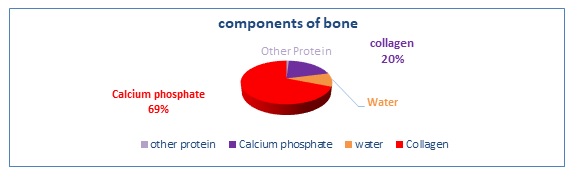
Figure 6. Pie chart of the natural component of human bone by density weight as a percentage
4.3.2. Anatomy of teeth
Teeth are the most inflexible and toughest parts of the body, and they have a distinctive component as well as a complex function [86]. The elemental composition of HAp is the same as the structure of the primary inorganic compound found in teeth. There were three distinct forms of hard tissue discovered in genuine teeth: (i) enamel is the strongest component, a primary structure including H2O, and organic element 3%, and HAp, 97%; (ii) cement is made up of organic matter and H2O 55%, and metals 45%; (iii) dentine is a chemical and biologically complex structure that is typically composed of 12%, 70%, and 18% water, inorganic, and organic matter [82, 86, 87]. The natural components of teeth, enamel, cement, and dentine, are depicted in the form of a pie chart in Figure 7. The density weight of each component is shown.
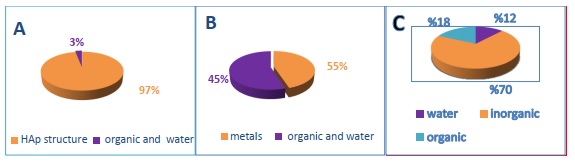
Figure 7. Component of teeth by density weight as a percentage: (a) Enamel, (b) Cement, and (c) Dentine
4.4. Mechanical behavior of HAp
HAp is rarely utilized outside of the realm of lightweight applications due to the poor mechanical qualities it possesses. The mechanical and physical properties of HAp are determined by several factors, including the Ca:P molar ratio, its microstructure, element shape, and size, solubility ratio, HAp specimen impurities, and the composition of the elements, which varies with phase [24, 82, 88]. The fracture toughness of the HAp ceramic was increased when it was heated to a temperature between 1373.15 and 1423.15 K. In addtion, the fracture toughness of the HAp ceramic did not change significantly when it was heated from 1323.15 to 1523.15 K. The fracture toughness of HAp heated at 1373.15 K is significantly lower than that of HAp heated at 1523.15 K. The TCP presence also contributes to a decrease in the fracture toughness of the material [24]. In high-density HAp, Young's modulus values are 35×103 to 120×103 MPa, very near to those of hard tissue. HAp compressive, bending, and tensile strengths are 120×103 – 900×103 MPa, 38×103-250×103 MPa, and 38×103- 300×103 MPa. These values are equivalent to those of hard tissue. The HAp strength increases in proportion to the Ca:P ratio, reaches its highest point at 1.67, and experiences a precipitous decline whenever the Ca:P ratio is lower than 1.67 as shown in Figure 8 [51, 82, 89].
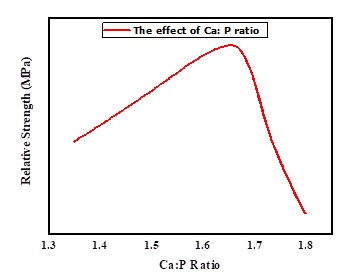
Figure 8. The effect of Ca: P molar ratio on the mechanical properties of HAp materials [18]
4.5. Dielectric property of HAp
The electrical dielectric property of pure and doped HAp has been the subject of investigation in a great number of investigations [40, 90-92]. One of the HAp properties of bioceramics is that they have a high dielectric constant. Temperature, the settings of the experiment, and the amount of impurity all has the potential to affect the dielectric constant (εr). The εr was raised at lowered frequencies as a result of polarisation and the quick realignment of dipoles with the external field. It may be possible to change the dielectric characteristics of HAp by manipulating the crystalline composition, particle arrangement shape, or size of the molecules. To evaluate r, researchers utilized the equation C×t/o×A. Polarisation and dipole deflection get interwoven when an external electric field is applied to HAp, which results in a change in the permittivity and AC conductivity of the HAp composition. The dielectric property may be of use in the treatment of osteonecrosis, bone growth, and bioactivity [68, 93, 94].
4.6. Optical property of HAp
When light travels through a variety of media, its energy undergoes transformations that cause phenomena such as transfer, scattering, and absorption. When it comes to the optical behavior of HAp, the effect is determined by the wavelength (λ), the refractive index (n), the εr value, and the growing impurity. Pure HAp has a n factor that ranges between 1.640 and 1.650, and this value is sensitive to polarisation as well as the way it transmits of light. The optical activity of the dopant HAp is dependent on the is used. To have an understanding of the optical properties of materials, one must have a working familiarity with concepts such as band gap energy, piezoelectric response, band structure, and other related ideas. The density functional theory (DFT) suggests that the energy band structure of HAp is somewhere between 5.40 and 4.50 eV, with the exact value depending on the valence state [52, 68, 92]. The optical properties of hydroxyapatite are closely tied to its crystal structure, the presence of impurities, and the methods used for synthesis. Researchers can tailor these factors to achieve specific optical properties for various applications, including in biomedical devices, coatings, and optical components.
4.7. Magnetic property of HAp
One other characteristic of HAp is that it has a diamagnetic behavior, and another is that it does not have any electrons that are unpaired in the d molecular orbitals (the electronic state) that it possesses [94]. Analyses of the magnetic properties of HAp using Fe2+, Sr, Co, Gd, and Fe3+ were the subject of a substantial number of studies carried out in times past [95, 96].
4.8. Thermal behavior of HAp
To prepare apatites for use in several applications, such as biomaterials, lighting, garbage disposal, and others, a specific technique that takes place at a high temperature is necessary. To accomplish this, you must have a deeper comprehension of the thermal stability as well as the phases that develop during the heating process. It is helpful to have an understanding of the temperature analyzing limit for different apatites; as a result, we will analyze the changes that occur in the substitutes' physical and chemical properties. HAp's thermal behavior is influenced by a wide range of synthesis-related characteristics, such as its structure, chemical composition, pH solution, crystallinity, mammographic ally, solubility in water, etc. [52, 97, 98].
4.9. Synthesis methods of hydroxyapatite powder
Although synthetic HAp and organic apatite have the same structure, organic apatite and synthetic HAp have very different physical, chemical, and mechanical characteristics. The following are the steps associated with making thick Hydroxyapatite:
- Creating apatite (HAp) powder or using commercially available apatite solutions
- Under great pressure or pressing into specific size and shape of crystal [1, 5].
To obtain HAp, it is possible to achieve metal components on Ca/P with various phases depending on the method, reagents, and variables used, resulting in materials with various compositions and properties, such as crystalline deformities, area of surface, organic compounds, etc. [99]. To obtain ceramic HAp biomaterial, many common methods have been discovered such as:
- Dry methods: Mechanochemical reactions, solid-state method.
- Wet chemical reactions: Hydrothermal reaction, precipitation techniques, and sol-gel method.
- Reaction at high temperatures: Thermal decomposition, combustion reactions [54].
Every one of the approaches for making HAp has various specific properties and can produce various shapes. The control of shape and size of crystals is one of the main challenges of synthetic hydrocarbon of HAp, this can have an impact on its mechanical qualities, preparation method, surface chemistry, and bioactivity [100, 101].
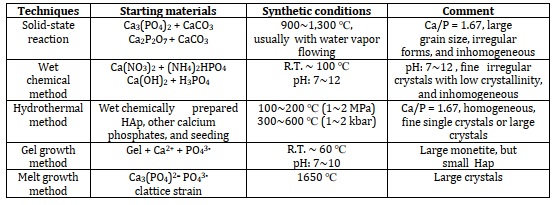
Table 5 shows that a lot of reactions produce a new calcium hydroxyapatite ceramic at temperatures between 950 to 1300 C. To get primarily HAp, β -TCP [Ca 3(PO4)2], or TCP, it is necessary to maintain control over both the pH of the biochemical reaction and the concentration of each of the components that react. Both a dense (microporous) and a macroporous version of TCP can be manufactured. Macroporous ceramic (pores > 500 μm) is prepared by adding foaming agents such as hydrogen peroxide or naphthalene before compacting, heating at low temperature to remove foaming agents, and then sintering at high temperatures (950-1100 C). Sintering at high temperatures (between 1100 and 1300 C) and pressures (between 140 and 200 megapascals), also known as hot isotropic pressing (HIP), is a form of hot pressing that can be used to create dense or microporous ceramics [102].
Table 5. Preparation techniques for HAp [55]
4.9.1. Dry method
A solvent is not necessary for dry techniques. Because they have little influence on the properties of the powders produced, these procedures are commonly utilized for the mass manufacturing of powders without the need for careful control of system parameters [67]. The reactions that take place in solid state among Ca2+ and P5+ compounds are exploited in dry techniques, which have the benefit of producing a mixture of HAp powders. The following solid-state reactions (Equation 1 & 2) can also be used to produce pure hydroxyapatite powder [1]:
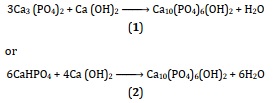
At 1200 °C, two major processes using water vapor will be used to synthesize HAp solid-state (Equation 3 & 4) [74, 79]:
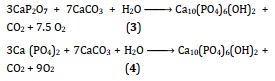
To increase the kinetic performance of the process of preparing HAp powder using a dry technique, mechanochemical synthesis has been utilized. In this case, precursor ion-containing materials are at the ground, and mechanical energy encourages structural alterations and chemical responses. As may be seen in the equations up top, it is possible to generate substituted apatites by utilizing the correct ratio of source materials in the production process.
4.9.2. Wet method
These methods make use of a variety of solutions, temperatures, and chemical precursors. Wet methods are a group of procedures for producing powders that involve occurring in the chemical reaction in the presence of a solvent system [104-106].
Chemical precipitation: is a method of production of HAp involves the use of aqueous solutions, where the chemical interactions among Ca2+ and P5+ ions happen at controlled pH and temperature. The most common reaction is neutralization, which produces water as a byproduct [42, 43]. Precipitated HAp is made by combining calcium nitrate (CaNO3)2 or calcium chloride (CaCl2) salts with ammonium hydrogen phosphate (PO4 -3) at pH values > 4.2, which is then corrected with concentrated ammonium hydroxide (NH4OH). The temperature of the reaction might range from room temp 25 C to approaching the melting point of the water. The following is an example of a general reaction (Equation 5) [107-109]:
10Ca (NO3)2 + 6(NH4) + HPO4 + 8NH4OH Ca10(PO4)6(OH)2 + 20NH4NO3 + H2O (5)
The basic processes for producing HAp particles by chemical precipitation are shown in Figure 9. In a nutshell, it entails the dropwise addition of one solution to another while maintaining the Ca/P molar ratio equal to 1.67 under continuous process and slow magnetic stirring [110].
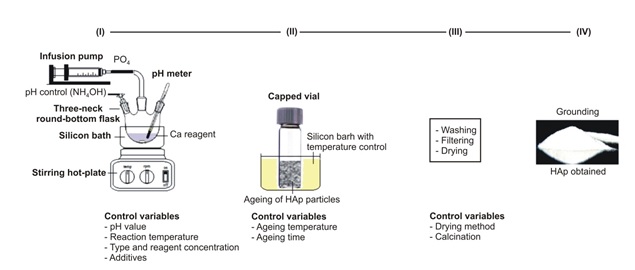
Figure 9. Chemical precipitation technique to hydroxyapatite synthesis diagram [67]
4.9.3. Sol-gel method
This method can provide physical and chemical properties of substances because of most importance and advantage. The sol-gel process includes two major mechanisms: (1) Precursor basic solutions or hydrolysis in acidic and (2) The hydrolyzed products' poly-condensation [111]. In most cases, (CaNO3)2 or CaO is reacted in an aqueous or organic solution with triethyl phosphate (C6H15O4P) or triethyl phosphite (C6H19O3P). The following Equation is a general reaction [112]:
![]()
That method has various benefits over previous processing methods, including a few temperatures, superior composition control, large purity, and the possibility to design methods for more application areas [113]. Figure 10 shows sol-gel hydroxyapatite production.
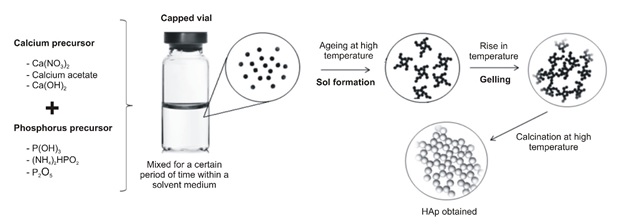
Figure 10. The sol-gel method for producing hydroxyapatite is depicted in this diagram [67]
4.9.4. Hydrothermal method
One of the common methods that can obtain hydroxyapatite is called hydrothermal at pressure and high temperature, with organic modifiers used to influence the form and structure of crystals produced [114, 115]. The formation of crystalline HAP can also be done by the process known as hydrothermal, which is a relatively developed technique. This method is the basic phrase that is employed to describe the reaction that takes place in the absence of the following: a calcium source; a phosphate precursor; (i) either water or an organic solvent, or (ii) a mixture of water and an organic solvent [116]. In the following reaction, HAp is happening [117]:
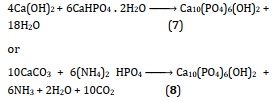
Due to the intermediate components of the predecessors phase, such as β -TCP, Ca3(PO4)2, tetracalcium phosphate (TTCP), Ca4P2O9, and Ca4(PO4) 2O, can be easily converted to HAp under hydrothermal conditions, this method makes it possible to obtain a high degree of crystallinity as well as a Ca: P ratio that is close to the stoichiometric value. The solid-state processes described above can also occur hydrothermally at a temperature of 275 °C and a steam pressure of 12,000 pounds per square inch (one pound per square inch is equal to 0.07 kilogrammes per square centimetre). The resultant HAp is frequently carbonate substituted, but it crystallises properly and is chemically consistent throughout. To prevent carbonate substitution, the system may be supplemented with the appropriate quantities of CaHPO4 or (NH4) 2HPO4, which will change calcium carbonate into HAp in the same manner described above.
4.9.5. Hydrolysis
The HAp powders can be made by hydrolyzing various calcium phosphates to obtain the starting material. As an illustration, a variety of calcium phosphates, such as dicalcium phosphate dihydrate (DCPD), (TCP, Ca3(PO4)2, OCP (Ca8H2 (PO4)6 • H2O, etc. can be hydrolyzed to produce a powder that is low in calcium and is called HAp. This type of approach stands out as particularly appealing for a number of different factors. It just needs low temperatures, which are generally less than 100 °C for manufacturing, it improves the physical characteristics of calcium phosphates by hardening, it utilises a single precursor, and it types calcium-deficient HA which is more dissolve than stoichiometric HAp and as a result, could be absorbed by bone more easily. These are some benefits that it provides. For instance, calcium-deficient HAp can be created using the following chemical reaction (Equation 9) [1]:
![]()
The hydrolysis product, on the other hand, is very nonstoichiometric and produces HAp needle or blades with a variety of sizes measured in microns. In addition, the traditional hydrolysis process at a low temperature can take anything from a few hours to several days to finish [1].
4.10. Biomedical application of HAp
Hydroxyapatite (HAp) is a mineral that is structurally equivalent to the mineral part that is found in human bone and other types of hard tissue, in the sense that it is composed of the same ions that make up the metallic component of teeth and bones. As a result, HAp has the same composition as the mineral component of bone and other hard tissue in humans [77].
- As a result of the exceptional osteoblast inductive characteristics that it possesses, HAp has discovered substantial potential as a bone substitute.
- The employment of HAp chemicals in clinical orthopedics for either tissue spacing or bone defect refilling is possible due to the important biological properties possessed by these compounds.
- The cement utilization in the fastening of HAp implant components makes it possible for the mitigation of problems brought on by high-density polyethylene wear elements.
- HAp is used in various biological applications, such as bone cement biometrics, bioavailability, toothpaste additives, and dental implants.
- HAp can be also used to make femur plugs for fully replaced hips [118].
- Coclusion
The field of biomaterials, particularly focusing on hydroxyapatite (HAp) as a prominent member, has evolved significantly over the last few decades. Biomaterials play a crucial role in bridging the realms of materials science and clinical medicine, serving as synthetic materials designed to replace living tissues and restore normal physiological functions.This comprehensive review has highlighted the diverse landscape of biomaterials, categorizing them into natural and artificial materials, and emphasized the significance of biomaterials in various biological applications. Among the artificial biomaterials, calcium orthophosphate-based bioceramics, particularly hydroxyapatite, have emerged as key players due to their structural similarity to bone and teeth minerals and their remarkable biocompatibility, thermal stability, and bioactivity.
While hydroxyapatite has found widespread use in biomedical applications, its limitations in load-bearing orthopedic applications due to poor mechanical properties have prompted extensive research into fabrication methods. The preparation conditions of synthesized hydroxyapatite significantly influence its physical and chemical characteristics, crystalline structure, and shape, making it a versatile material adaptable to specific medical applications.
To sum up, the study underscores the vital role of biomaterials, with a particular focus on hydroxyapatite, in advancing medical science and improving human health. The ongoing research and development in the field of bioceramics continue to contribute to our understanding of materials science and its applications in clinical medicine, promising innovative solutions for the repair and replacement of hard tissues in the human body.
Acknowledgements
Authors would like to thank everyone that contributed to the success of this review article and also the Journal of Chemical Reviews for their unstinting efforts.
Orcid:
Rebaz Obaid Kareem: https://orcid.org/0000-0001-6273-1309
Niyazi Bulut: https://orcid.org/0000-0003-2863-7700
Omer Kaygili: https://orcid.org/0000-0002-2321-1455
Citation: R.O. Kareem*, N. Bulut, O. Kaygili, Hydroxyapatite Biomaterials: A Comprehensive Review of their Properties, Structures, Medical Applications, and Fabrication Methods. J. Chem. Rev., 2024, 6(1), 1-26.


