Document Type : Review Article
Authors
1 Department of Chemistry, Hazara University, Mansehra, 21300, Pakistan
2 Nanoscience and Technology Department, National Centre for Physics, Islamabad, 44000, Pakistan
Abstract
The existence of the carcinogenic agent hydrazine poses a serious hazard to environmental wellbeing. Consequently, effective hydrazine detection in aqueous conditions becomes crucial. Novel sensing electrodes are being created through modifications using conducting polymers and nanomaterials, such as carbon-based nanomaterials, metallic nanoparticles, and metal oxide nanoparticles, in order to improve the selectivity and sensitivity of hydrazine detection. This review article offers a thorough assessment of the most recent developments in conducting polymer nanocomposites-based electrochemical sensing electrodes for hydrazine detection. These innovative electrodes are made to keep low detection limits while providing better sensitivity, selectivity, and durability. The review intends to provide information about the creation, evaluation, and performance of the sensing electrodes as well as their potential for practical use.
Graphical Abstract
Keywords
- Electrochemical sensors
- Hydrazine
- Conducting polymers
- Nanocomposites
- Nanotechnology, Voltammetric sensor
Main Subjects
- Introduction
Ecological contamination is one of the major issues our society is confronting. The rapid intensification in the levels of environmental pollution over recent decades has resulted in growing concern for human health and overall ecosystems [1]. The primary purpose of increasing dangerous toxins in the environment is caused by human activities and various industrial processes [2, 3]. As a result, people are exposed to harmful chemicals through several sources [4, 5]. A significant class of toxic pollutants includes heavy metals, fluorinated carbons, and organic and inorganic pollutants [6, 7]. These pollutants have harmful effects on health, triggering some severe problems, and prolonged exposure to these pollutants can be life-threatening [8, 9]. Hydrazine (N2H4) is a vital laboratory and manufacturing chemical discovered by German scientists. Hydrazine is commonly used in numerous fields, such as catalysis, medical, chemical, fabric dyes, farming industries, and as a monopropellant and bipropellant rocket fuel in aerospace industries [9-11]. Even though hydrazine (HZ) has various uses, prolonged exposure can harm one’s health. Several industries release HZ in vast amounts from where it can enter the drinking water supply every year. It is carcinogenic, neurotoxic, and can harm the liver, kidneys, brain, and other vital organs of the human body [12]. According to the World Health Organization (WHO), hydrazine is categorized as B2 carcinogenic [13, 14]. Early HZ detection in aqueous media is essential and critical. Therefore, the hydrazine sensing at a low concentration has been the central area of research in the last few years [15]. Hydrazine is detected using various analytical procedures, including colorimetry, chromatography, fluorescence, chemiluminescence, titration, and electrochemistry [16, 17]. The electrochemical system has several advantages over other approaches, including ease of use, lower costs, transferability, quick processes, high selectivity, in situ reductions, and an extensive measurement range, making it more prominent among the available techniques. Electrochemical sensors were first used in the 1950s, and since that, electrochemistry has made significant advances. The progress in electrochemical engineering and new electrode materials have offered possible practical and appealing solutions [18, 19]. The performance of the electrochemical sensor is related to the material of the working electrode [20], and it has made significant progress in recent years by improving the detecting capabilities of the working electrode [21]. The revolutionary work of Alan J. Heeger, Alan G. MacDiarmid, and Hideki Shirakawa, for which they were awarded the Nobel Prize in Chemistry in 2000, is credited with the first use of conducting polymers in electrochemistry. They discovered that when some organic polymers, notably polyacetylene, were chemically doped, they may display electrical conductivity. Their discovery was significant because it called into question the prevailing wisdom that only metals and inorganic materials could successfully conduct electricity. Conducting polymers opened up new avenues for the production of lightweight, flexible, and simply processable electrically conductive materials. A key issue in electrochemical sensor design is understanding the surface structure and reactivity. Understanding the fundamental processes that influence sensor response leads to the creation of electroanalytical devices with more excellent sensitivity, selectivity, high stability, and lower detection limits in most circumstances. Thus, rapid and reliable electrochemical sensors detecting low concentrations can improve real-time environmental monitoring [22, 23].
Conducting polymers (CPs) have been investigated extensively used as a transducer in electrochemical sensors to enhance speed and sensitivity, and they are proving to be quite effective [24]. The properties of CPs like tunable conductivity, facile synthesis, and easy modification, environmentally friendly, intensely sensitive to a wide range of HZ at ambient temperature, and economical make them the most appropriate material for use in electrochemical sensing [25]. By incorporating functional nanomaterials into conducting polymers, it is possible to efficiently overcome the constraints of these polymers in their natural form, notably in terms of electrochemical sensing. Nanomaterials have distinct physical and chemical properties that make them ideal for sensor applications [26]. The general properties of conducting polymers can be considerably improved by introducing nanomaterials such as carbon nanomaterials and metal/metal oxide nanoparticles as dopants. The nanoparticles incorporation into the composite material has various advantages. First, nanoparticles have increased electrical conductivity, allowing for better conductivity in conducting polymers. Second, their increased surface area improves the interaction between composite material and the analyte in electrochemical sensing. Finally, nanoparticles have superior electrochemical activity, which leads to improved sensor performance. [27]. They have higher electrical conductivity, a bigger surface area, and better electrochemical activity [28]. As a result, electrochemical sensors that have been enhanced with conducting polymers and nanomaterials excellent conductivity, sensitivity, selectivity, and solid adsorption capabilities. The synergistic benefits of combining conducting polymers and nanoparticles in nanocomposites have the potential to revolutionise the area of electrochemical sensors [29]. The synergy between CPs nanocomposites is expected to bring exciting advantages in electrochemical sensors [30, 31].
In the recent past, many review papers have been published on electrochemical sensing of hydrazine targeting one specific material of interest, thus leaving a critical and primarily used class of materials, conducting polymers, and their nanocomposite. Therefore, it is essential to mention the range of conducting polymers and nanomaterials deployed for HZ electro-oxidation. Research is still required on the fundamental interactions of HZ with various CPs/nanomaterials, which could have promising implications in HZ detection. This review study discusses the sensing properties of conducting polymers and the effects of nanofiller dispersion and compatibility on nanocomposite properties, including the oxidation mechanism and pH factor for identifying, analyzing, and monitoring the most dangerous chemical hydrazine. This article provides platforms for new conceptual frameworks, and constructs diverse results. It is important to note that different analytical terminology is used in the later section of this review. Readers need to learn and grasp these terms used in studies. Various hydrazine analyses are performed every year, and conclusions are drawn based on the findings. Following the calculations and decisions, it is critical to guarantee that the method carried out produces the intended precise outcome with more precision. Therefore, method validation is the process of establishing written evidence that a test procedure fits the intended purpose in terms of quality, reliability, and consistency of results. A few important characteristics of method validations are displayed in Figure 1.
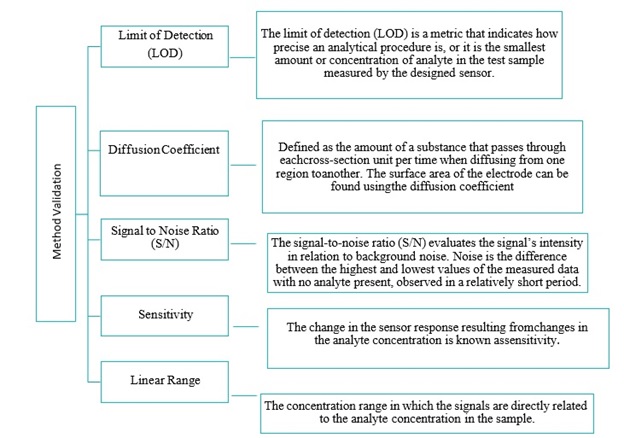
Figure 1. Definition of few important method validation parameters used in analysis of hydrazine
- Electrochemical Approach for Sensing and Challenges in Electrochemical Sensing
Due to the sheer surge in pollution, sensors have become more important in protecting the environment [31]. Every sensor comprises a transducer that converts chemical inputs into electrical signals and a chemical interface. The analyte interacts chemically with the surface, causing a change in physical or chemical properties, and the transducer is a device that responds to a specific analyte by providing output. Sensor key benefits are ease of use, small size, and likely low cost [32]. An electrochemical sensor is a tool that alters electrochemical data into an analytically convenient signal involving a free electron transfer between an electrode and a phase that may be liquid or solid [33, 34]. The electrochemical setup consists of a counter (CE) electrode, a reference (RE) and a working electrode (WE). The counter electrode (CE) offers electron flow to complete the circuit. A platinum wire is mainly used as the counter electrode. In contrast, the reference electrode provides stable voltage within the electrochemical cell. The reference electrode is usually comprised of Ag/AgCl. The working electrode is the essential component of the whole setup, at the surface of working electrode, a reaction of interest occurs. Electrochemical sensing is based on the oxidation of hydrazine. They are generally executed by monitoring the working electrode’s potential at a static value and observing the current as a function of time. The current response reveals the HZ concentration as it goes through the sensor. Primarily used analytical procedures for HZ include voltammetry, chronoamperometry, and potentiometry. Cyclic voltammetry, square wave voltammetry, and differential pulse voltammetry have all been widely used among these approaches. The defining feature of cyclic voltammetry, a widely used electrochemical sensing method, is a prominent oxidation peak. It reflects the oxidation of a molecule electrochemically at a particular voltage. It is a valuable instrument for quantitative investigation because the height of the peak is related to the concentration of the molecule being oxidised. In cyclic voltammetry, the term "scan rate" describes the pace at which the potential is swept. It influences the size and shape of the oxidation peak and can be utilized to enhance the measurement's sensitivity and resolution. The properties of the electrochemical sensor mainly depend upon the material of the working electrode [35]. However, orthodox bare electrodes unveil low sensitivity, selectivity, poor reliability, sluggish electron transfer kinetics, and high over potential (requiring more energy to drive the reaction) for the oxidation of hydrazine [36, 37]. Complex matrices, intrusion from co-existing species, poor detection limits, stability issues, calibration issues, and high costs are some of the obstacles unique to electrochemical sensing. These difficulties may reduce the sensor response's accuracy and dependability. To circumvent these challenges, conducting polymers and nanomaterials are being employed to change the interface of electrochemical sensors [38, 39]. The use of conducting polymers, metals, metal oxide, and carbon nanoparticles-based electrodes has additional benefits, such as lowering the over potential for hydrazine oxidation and speeding up interfacial electron flow between hydrazine and electrode surface providing high sensitivity and selectivity [40, 41].
- Electrochemical Oxidation Mechanism of Hydrazine on Surface of Working Electrode
The working electrode surface is used as the reaction site to understand the charge transport properties of CPs nanocomposite when exposed to HZ [42]. It is crucial to learn about the surface reaction of an electrode since it affects the rate as well as the amount of electron transport between the electrode and the solution. The kinetics of the process, such as the pace at which ions diffuse to the electrode surface and the rate at which electrons pass between the electrode and the solution, are also influenced by the nature of the reaction. Therefore, optimizing electrochemical processes and building effective electrochemical systems require a thorough understanding of the surface reaction. The system's electrochemical behavior, including the electrode's potential and the surface current density, is likewise impacted by the surface response. It is possible to learn more about the underlying electrochemical mechanisms and the surface reaction by examining it. The HZ oxidation has been studied extensively on several electrodes to understand the electro-oxidation reaction mechanism. Ruiyang Miao et al. investigated the oxidation mechanism of HZ and found that it is a four-electron transfer process with the release of nitrogen and protons. Adsorption of hydrazine molecules onto the electrode surface is the initial step in the electrochemical oxidation of hydrazine. It is revealed that the unprotonated form of HZ (N2H4) is only electroactive while the N2H5+ (hydrazinium ion), which is protonated form, is electro-inactive [43]. The current build after the reaction is observed and used to calculate important data such as concentrations from the sample. The mechanism specifics can change based on the experimental parameters and the electrodes characteristics. To create the electrochemical sensors for hydrazine detection and similar applications, it is essential to comprehend this mechanism. Adel A Ismail et al. also studied the hydrazine oxidation mechanism on mesoporous Au/ZnO nanocomposite electrodes. The electro-oxidation reaction of hydrazine was determined and calculated, revealing a four-electron transfer process with the release of nitrogen (N2) gas as an end product [44]. Similar studies carried out by various researchers [45-48] unfold the exact oxidation mechanism. Therefore, it is commonly accepted that the N2H4 oxidation is a four-electron transfer procedure with nitrogen discharge. The reaction mechanism is given below. The electrochemical oxidation of hydrazine follows a complicated mechanism that involves the creation of numerous intermediates. However, the design of hydrazine-based industrial processes and the creation of effective electrochemical sensor depend on a thorough understanding of this mechanism.

As discussed earlier, the rate of diffusion and electron transfer in hydrazine oxidation is affected by a number of parameters, including the type of the electrode, the concentration of hydrazine in the solution, and the presence of other species in the solution. Numerous variables, such as the electrode surface's composition, the solution's pH, and the presence of other species, might affect the kinetics of hydrazine oxidation. For instance, the surface area of the electrode, the hydrazine's diffusion coefficient, and the pace at which the solution is stirred can all affect how quickly hydrazine diffuses to the electrode surface.
- Effect of pH on Hydrazine Oxidation
The HZ oxidation is a pH-dependent procedure and a crucial factor to understand as it affects the HZ oxidation current and potential. The pH value was evaluated and optimized in numerous studies to enhance sensitivity [49, 50]. A buffer solution can be employed as an electrolyte in electrochemical sensing to give the electrochemical process a stable pH environment. To achieve precise and trustworthy measurements, it is crucial to keep the pH constant. The buffer solution steps in at this point. The change effect in pH on the oxidation of hydrazine was determined by Marya Khan et al. detected hydrazine on the ZnO nanosheets-based FET sensor. The targeted range of pH range was 5 to 9. The response of hydrazine sensor improved significantly as the pH of the buffer solution was raised from 7 to 8. Given that the pKa value of hydrazine is (pKa =7.9) when the pH was near to the pKa value, HZ was in a neutral state, making oxidation easier, and providing optimum sensing response. At pH> pKa, hydrazine converted into its deprotonated form, which hindered its oxidation and resulted in a lower peak current. Hence the optimum pH for maximum current sensitivity was found to be 7.4 [51]. Seul Ki Kim found a pH effect on hydrazine oxidation by changing pH from 5 to 10 [52]. The pH effect was also studied by Fugang Xu et al. with different pH values of PBS buffer from 5 to 10. Electric current builds as the pH increases from 5 to 6, and an oxidation peak appears. When pH changes from 7 to 10, an increase followed by a decrease in the current is observed. The primary response occurs at pH near the pKa value of HZ. This confirms that current shifts at various pH values are linked to hydrazine pKa. The HZ becomes protonated when the pH of the PBS buffer solution falls below pKa, resulting in a mild oxidation current [53]. As a result, the ideal pH for phosphate buffer solutions was determined to be >7, and it is frequently employed in the electrochemical detection of hydrazine.
- Role of Conducting Polymers and Their Sensing Mechanism
Conducting polymers (CPs) are a type of organic molecule with many applications in electrochemical sensors because of their underlying physicochemical attributes [55]. Different kinds of CPs, as depicted in Figure 2, include PPY (Polypyrrole), PANI (Polyaniline), polythiophene (PTh), poly (3,4-ethylene dioxythiophene) (PEDOT), and poly(3-hexylthiophene) (P3HT) have structural characteristics, high conductivities, fast response time, good sensitivity, and a selectivity towards analytes which make them the most appropriate materials used in electrochemical sensors [56]. Conducting polymer sensing mechanisms can involve oxidation-reduction reactions, adsorption, and desorption of the analytes ions [57]. When a CP is added to a solution containing target molecules, its charge transport capabilities change (movement of electric charge from one end to the other in the electrochemical cell), affecting the CP conductivity, which can be evaluated using electrochemical techniques. Understanding the charge transport properties of electrochemically active CPs nanocomposites can be interesting. Conducting polymer has a backbone of π electron system responsible for their conductivity [58]. Though the π electrons delocalization (alternating single and double bond) along the polymer chain is not enough to acquire high conductivities, a doping process is required to enhance the conductivity of polymers [59]. Increasing the doping level increases more charges in the polymer and, consequently, outcomes of better conductivity [60]. The doping process of conducting polymer is achieved by the protonation of nitrogen atoms present in the polymer structure [61]. It is indeed possible to alter the properties of CPs by hybridizing them with different materials, which as a result, enhances the main polymer chain performance. Therefore, it is feasible to make a CPs composite out of highly conductive nanoparticles. The choice of nanoparticles is critical for achieving the desired nanocomposite characteristics. For the desired qualities, the nanofiller type, shape, and surface area must be managed [62]. The final nanocomposite features may also vary due to the nanoparticles interaction with polymer matrix. Another essential aspect of the polymeric nanocomposite is the nanoparticles dispersion in the matrix to further strengthen its inclusive sensing properties. The even distribution of nanoparticles throughout the matrix improves sensitivity and selectivity [63]. Furthermore, disseminating nanoparticles within the polymer inhibits nanoparticle aggregation, resulting in a larger surface area. The most widely used nanoparticles are metal/metal oxide nanoparticles and carbon nanoparticles (S/MWNTs, Graphene) [64].

Figure 2. Structures of some important conducting polymers [86]
Conducting polymer nanocomposites has a huge influence on the electrochemical sensor research; they demonstrate superior performance to conducting polymers in bulk due to the large surface-area-to-volume ratio [65-67].
- Nanoscale Carbon-Based Materials
Carbon nanomaterials (CNMs) have become a popular material for electrochemical sensors in recent years. The extraordinary properties of two allotropic forms of CNMs, i.e. Carbon nanotubes (CNTs) and Graphene makes them the most appropriate material for electrochemical applications due to the large surface area, high electrical conductivity, and effective electrocatalytic behaviour as they possessed sp2 hybridized structures Figure 3 [69, 70]. When dispersed in the CP matrix, CNMs significantly increased the rate of chemical oxidation because of their greater surface areas [71, 72]. CNTs have high aspect ratios and ID structures, while graphene possesses a 2D structure, and both show high sensitivity towards any changes in their chemical surroundings [73, 74]. Moreover, their electron transport properties also make them suitable materials for sensors [75]. Graphene (2D) and various types of graphene (Nano flakes, Nanoplatelets, Reduced graphene, Oxidized graphene, etc.) are the ideal materials for electrochemical sensing [76, 77]. Their exceptional properties (conductivity, large specific surface area, high sensitivity and selectivity, low detection limits, and durable stability) make them essential for modifying electrode materials to detect hydrazine at low concentrations [78]. Furthermore, chemical modification is a common way to enhance the CNTs properties and graphene [79, 80]. The existence of reactive groups on the CNTs and graphene surface permits them to be electro-catalytically active. Incorporating carbon nanomaterials in the polymer matrix with improved dispersion and strong adhesion are severe issues in gaining further improved properties [81]. Incorporating CNMs in conducting polymers significantly increases their electrical conductivity by several magnitudes. The enhanced conductivity can increase the electron transfer rate in electrochemical sensors depending on the CNPs dispersion and aspect ratio [82-86].

Figure 3. Diagram of multilayer graphene and carbon nanotubes [87]
- Metal/Metal Oxide Nanoparticles
Metal nanoparticles have drawn much interest because their inherent size-dependent properties differ from comparable bulk materials [88]. They have vital applications in catalysis and sensing. Metal nanoparticles like gold, silver, copper, cobalt, iron, etc. possess a large surface area to volume ratio, higher electron transfer rate, large surface energies, and chemical modification [89, 90]. They are massively used as a nanofiller in conducting polymer matrix design for environmental applications. The size, shape, and chemical properties of nanoparticles (NPs) utilised in polymer materials vary. These NPs have the potential to drastically alter the properties of the resulting polymer materials by influencing surface chemistry, physical complexity, and chemical structure. The properties of polymeric nanocomposites are determined by the interactions between the NPs and the polymer matrix. When nanoparticles (NPs) are dispersed in a polymer matrix, they interact which can change the polymer's behaviour, shape, charge distribution, and bond dispersion. Finally, the NPs incorporation into polymer materials can result in significant improvements in performance characteristics. The metal nanoparticles can be protected and stabilised by the polymeric matrix, which can further stop aggregation and degradation. This could increase the electrochemical sensing system's durability and reproducibility. [91, 92]. As a case in point, Chanaka Sandaruwan et al studied the effect of palladium nanoparticles by dispersing them in a polyaniline matrix for sensing application. The study's findings showed that the addition of Pd nanohybrids significantly affected PANI's capacity for sensing. In particular, compared to pure PANI, the PANI/Pd nanohybrids showed improved sensitivity and selectivity toward moisture and hydrogen detection. There are several explanations for the nanohybrids enhanced sensory abilities. The Pd nanoparticles, first and foremost, function as catalytic sites that encourage the dissociation of water molecules and hydrogen molecules. This makes it easier for them to interact with PANI, which causes a stronger reaction. Additionally, Pd nanoparticles increase the nanocomposite's surface area and conductivity, improving its sensing capabilities
Metal oxide nanoparticles have been used in sensing applications since the early 1990s and have gained much attention in the electroanalysis of HZ [93]. They have been integrated into conducting polymer matrix due to their better electrocatalytic, thermal, and chemical properties [94, 95]. The conducting polymer and metal oxide nanocomposite form heterojunction that can be very sensitive to the analyte [96, 97]. Such unique characteristics of metal and metal oxide nanoparticles make them desirable for use as reinforcement in polymer composites. Furthermore, leaching or degradation of the polymer chains of conducting polymers can reduce their stability and long-term performance. Metal, metal oxide, and carbon nanomaterials can give the polymer matrix mechanical support, preventing the detachment or dissolution of the polymer chains. Moreover, they can shield the polymer from elements that could eventually cause it to decay, such as moisture, oxygen, and the UV light. Longer sensor lifespan and dependable sensing performance are made possible by the nanocomposite's improved stability.
- Binary Composites of Conducting Polymers Composites for Hydrazine Sensing
Materials made of two different components are referred to as binary composites. The nano filler’s dispersion and the interfacial linkage between filler and polymer matrix are vital to improving the polymer matrix’s sensing properties. Nanofiller have diverse properties, and their addition to conducting polymer leads to the effectiveness of CPs properties [98-100]. The advanced electrode based on binary composition for the electrochemical sensing of hydrazine is discussed here.
8.1 Silver nanoparticles-CPs
Silver nanoparticles (AgNPs) have high conductivity and excellent electrocatalytic properties than other metal nanoparticles [101]. It is the favorite material to be incorporated in a conducting polymer matrix to enhance its overall sensing properties [102]. The potential of silver nanoparticle-conducting polymer nanocomposites in sensing applications has been thoroughly investigated. These substances combine the electrical conductivity of polymer matrix with the large surface area and catalytic capabilities of the silver nanoparticles to produce a material that is extremely sensitive to the presence of analytes. Singh et al. prepared a binary nanocomposite of AgNPs/polyaniline by photolysis of aniline to polyaniline deposited in indium tin oxide surface (ITO). The synthesized nanocomposite was used as the electrode material to detect low concentrations of hydrazine. The solution was kept under the UV light at a specific wavelength for 12 hours during the in situ polymerization process. ITO/PANI/AgNPs electrode was tested for hydrazine detection using Differential pulse voltammetry (DPV). The DPV technique was carried out in 0.1 M PBS (pH=7.0), revealing an enhanced peak at 0.12V. The HZ concentrations were used from 0.0010-0.50 mM with an increase in concentration, the increase in the corresponding peak was observed, revealing the sensitive nature of prepared nanocomposites due to the nanofiber morphology of the PANI matrix and the presence of silver nanoparticles in the matrix [103]. P. Paulraj et al. attempted to incorporate silver NPs in the polyaniline matrix by interfacial polymerization. The prepared nanocomposite was used as an electrode material for the electrocatalytic detection of hydrazine at very low concentrations using a glassy carbon electrode. The electrocatalytic oxidation of hydrazine revealed a better response towards hydrazine sensing showing the hydrazine potential at 0.4 V with a more significant oxidation peak current in PBS sol at 8 compared to bare glassy carbon electrode. By further increasing the hydrazine concentration, from 20 µM to 80 µM, the increase in oxidation current peak was observed confirming the sensitive nature of the modified electrode [104]. Ghanbari et al. (2014) prepared binary nanocomposite in two steps by electrodepositing silver nanoparticles on polypyrrole (PPy) nanofiber on a glassy carbon electrode (Ag/PPy/GCE) for HZ sensing. The AgNPs were dispersed uniformly in the polymer matrix to achieve high electrocatalytic properties. The cyclic voltammetry (CV) method was deployed to study the electrocatalytic behaviour and electron transfer rate of PPy/Ag nanocomposites in 0.01 M HZ and 0.1 M Na2SO4 solution. The 64.5 µA mM−1 of HZ oxidation current was observed for 0.0005-0.001 mM and 11.4 µA mM−1 for 0.001 to 0.01 mM concentration. The detection limit for HZ concentration was found to be 0.20 µM. Moreover, chronoamperometry was deployed to find the diffusion coefficient of the working electrode. Hydrazine’s diffusion coefficient was calculated to be 2.64 ×106 cm2.s1. The sensor also has outstanding selectivity, reproducibility, and stability. Arguably, silver nanoparticles catalytic activity in hydrazine detection entails boosting surface adsorption, promoting electron transfer and speeding up the oxidation reaction. When silver nanoparticles are included in the conducting polymer matrix, these catalytic capabilities help to improve sensitivity, lower detection limits, and more effectively detect hydrazine. The combined synergic effect of PPy nanofibers and silver nanoparticles proven as substantial electrode material for HZ sensing. Therefore, the use of AgNPs in the conducting polymers matrix is endorsed as a positive selection towards the development of a novel class of electrode materials for hydrazine detection [105]. It is particularly interesting because it shows that HZ molecules are diffusing quickly through the solution, which is crucial for the accurate and precise detection of HZ. It is crucial to keep in mind that a number of variables, including the size and shape of nanoparticles, the concentration and pH of the electrolyte solution, and the potential scan rate used in the cyclic voltammetry experiment, may have an impact on the electrocatalytic behaviour and electron transfer rate of PPy/Ag nanocomposites. To completely comprehend the electrocatalytic behavior of PPy/Ag nanocomposites and to improve their functionality for HZ detection, more research is required.
8.2. Gold nanoparticles-CPs
The distinctive physical and chemical attributes of gold nanoparticles (AuNPs) make them exceptional scaffolds incorporated in the CPs matrix to fabricate novel electrochemical sensors. Due to their small size, gold nanoparticles have a high surface-to-volume ratio. These nanoparticles greatly increase the surface area that is open to electrochemical reactions when they are added to the CPs/Au electrode. This greater surface area offers more catalytically active sites, increasing the electrode's overall sensitivity. Furthermore, gold nanoparticles have exceptional abilities for transferring electrons. The presence of free electrons on their surface gives them a high density of conduction electrons. During catalytic processes, these electrons enable effective electron transport between the electrode and the molecules of the analyte. The CPs/Au electrode thus experiences faster and more effective electron transport kinetics, increasing sensitivity [106]. Xin et al. (2014) have used gold nanoparticles/PANI-based nanocomposite for hydrazine detection. Gold nanoparticles were synthesized without any reductant and auto-formed during the adsorption process of PANI and AuCl-4, where PANI acts as both the reductant and supporting agent. CV was performed in PBS pH=7.0 and measured the catalytic activity of PANI/Au toward hydrazine oxidation resulting in a very sharp and enhanced peak compared to the PANI films. DPV curves for hydrazine concentration in PBS pH 7.0 at different rates showed a linear response at 0.01 mM to 6 mM, as illustrated in Figure 4. The lower detection limit was found to be 1 µM. The increase in sensitivity and selectivity of the PANI/Au electrode can be attributed to the better catalytic properties of gold nanoparticles [107].

Figure 4. Representation of the preparation process to PANI/Au0 nanocomposites. DPV slopes of various hydrazine concentrations at (PANI/Au0)/GCE in pH 7.0 sol [107].
Gutiérrez-Pineda et al. have prepared gold nanoparticle decorated polypyrrole (PPy)/stainless steel electrodes. PPy films were initially produced by electrochemical polymerization, leading to the deposition of gold nanoparticles. The voltametric investigation executed at 0.050 V.s−1 display that the HZ electrochemical oxidation occurs on Au/PPy/SS electrode at far lesser anodic over potentials than the bare electrode. The better electrochemical activity of the AuNPs/PPy electrode in parallel to a gold electrode specifies that AuNPs/PPy increases the electrode’s surface area and acts as a novel electrochemically active sensor for HZ [108]. Oukil et al. synthesized gold nanoparticle/polypyrrole deposited on the iron electrode. The electrode was tested for hydrazine sensing and showed an excellent response for HZ oxidation by cyclic voltammetry. The designed electrochemical sensor revealed a sensitivity of 0.05 μA/μM and a detection limit of 6 × 10–3 mM towards hydrazine, disclosing the high catalytic properties of the electrode. The synergistic effect that results from combining AuNPs and CPs in a nanocomposite can increase the sensitivity and selectivity of electrochemical sensors. The CPs can function as a supporting matrix, providing stability, and improving the kinetics of electron transfer, while the AuNPs can act as nanoelectrode, offering a significant electroactive surface area for redox processes.
Therefore the electrode surface made of CPs and gold nanoparticles makes it easier to detect hydrazine electrochemically. With the aid of CPs/Au as a catalyst, hydrazine molecules adhere to the electrode surface, undergo electrochemical oxidation, and then create a detectable current response. High stability, conductivity, and catalytic activity of the composite allow for sensitive and targeted measurement of hydrazine concentrations.
8.3. Palladium nanoparticles-CPs
Palladium nanoparticles (PdNPs) are an effective electrocatalytic material for electrochemical sensors due to their increased surface area over the bulk metal [109]. The electrochemical oxidation, adsorption, and subsequent detection of hydrazine are all made possible by the employment of Pd NPs and CPs on the electrode surface. Using both the special qualities of palladium nanoparticles and conducting polymers, the composite improves the sensitivity and selectivity of hydrazine sensing. Svetlozar et al. deposited palladium nanoparticles in polyaniline by layer technique. PdNPs–PANI nanocomposites were subjected to electrocatalytic sensing of hydrazine. The concentration-dependent voltametric currents were observed in the concentration range of 40-800 µM HZ. The sensitivity increase was observed with the quantity of adsorbed Pd NPs. Furthermore, the amperometry result indicated a rectilinear response in the 10-300 µM range, and the sensitivity and detection limit was evaluated to be 0.5 µA/µmolcm−2 and 0.06 µM. The proposed methodology delivers the prospect of attaining a high ratio of electrically interactive metallic NPs within the CPs matrix to achieve better results [110]. Veniamin and companions (2013) dispersed palladium nanoparticles in conducting polymer poly-3, 4-ethylene dioxythiophene (PEDOT) matrix. Electrocatalytic properties were studied by amperometry for hydrazine concentration. The sensors offered a detection limit (LOD) of 0.8 µM and a linear range of 0.5-30-200-5,000 µM. The amperometric response shows the upsurge in the sensitivity of modified electrode towards hydrazine due to the large surface area of palladium nanoparticles [111]. Elena G. Tolstopjatova et al. prepared poly (3, 4-ethylene dioxythiophene) and poly (styrene sulfonate) (PEDOT-PSS). The PEDOT-PSS with Pd nanoparticles was drop cast on a glassy carbon electrode. Cyclic voltammetry and chronoamperometry were utilized as the primary tool to check the electrochemical properties of the metal-polymer composite, showing a response to hydrazine concentration from 0.4 to 100 μM. Different numbers of PEDOT: PSS/Pd layers were deposited on the electrode surface to evaluate their performance. The electrode with high Pd content shows higher sensitivity. The limit of detection, LOD=0.12 μM, and the maximum sensitivity of 14 μA μM−1cm−2 were obtained towards the hydrazine. The rate of hydrazine detection increases with increasing palladium NPs loadings showing that electrocatalytic performance is controlled by Pd NPs [112]. Moreover, this class of electrodes exhibited significant stability. Pd/CP nanocomposite as electrode materials demonstrated successful electrocatalytic activity, indicating that they have potential applications in electrochemical sensing.
8.4. Carbon nanomaterials/CPs binary nanocomposite
Carbon-based materials show numerous benefits such as low manufacturing cost, immense surface area, chemical stability, and exceptional conductivity [113]. This nanocomposite-modified electrode surface has unique features that allow for the detection and measurement of hydrazine. Tzu-Yen et al. have prepared nanosheets of reduced graphene oxide (rGO) and poly (3, 4-ethylene dioxythiophene) nanotubes (PEDOT-NTs) intended for electrochemical detection of hydrazine. Employing cyclic voltammetry, the electrochemical activity of a bare GCE electrodes modified with PEDOT NTs, rGO, and rGO/PEDOT NTs towards hydrazine oxidation were assessed. The rGO/PEDOT NTs nanocomposite compared to bare GCE, RGO, and PEDOT NTs showed a much higher oxidation current. The higher catalytic current indicates that the enhancement is accredited to the more excellent electrocatalytic activity and the higher surface area of rGO/PEDOT NTs. Thus, a sensitivity of 664.7mA mM-1.cm-2 and a limit of detection (LOD) of 2.2 mM showed an excellent response toward hydrazine detection [114]. Ameen et al. prepared a modified electrode based on polyaniline/graphene (PANI/Gr) composites. The response of PANI/Gr electrode current-voltage (I–V) plots were recorded in the concentration range of 0.01 µM–0.1 mM, as shown in Figure 5. The sensitivity and detection limit of∼32.54×10−5 A cm-2.M−1, ∼15.38 mM was noted with a correlation coefficient (R) of 0.78578 and a short response time (10 s). The electrochemical oxidation mechanism of hydrazine is given in the following reaction.
![]()
It has been revealed that the PANI/Gr electrode has an excellent resolution, making it suitable to detect HZ in real samples [115]. Hence, carbon nanomaterials/conducting polymer nanocomposites with a high affinity for HZ molecules are effective materials (Table 1).

Figure 5. I–V chart of PANI/Gr composite film in HZ concentrations of 0.01 µM-0.01 M in 10 ml of 0.1 M PBS solution 115.
Table 1. Literature comparison of various CPs Binary Composite Electrode for HZ sensing

8.5. Metal oxide NPs-CPs
Metal oxide nanoparticles also unveil fascinating features such as size controllability, great chemical strength, and easy fabrication with easiness of surface modification, capability to stimulate the electron-transfer rate, and electrocatalytic effect [119]. Recently, highly sensitive novel electroanalytical sensors made up of nanostructured metal oxides-CPs are economical with greater precision when tested to hydrazine molecules [120].
8.6. Zinc oxide NPs-CPs
Zinc oxide nanoparticles are distinctive inorganic semiconductor metal oxide with a wide bandgap of 3.37 eV. It is now widely used in electrochemical applications due to its environmentally free nature and its odd electrochemical properties [121]. Faisal et al. have prepared a polythiophene-based ZnO nanocomposite for the electrochemical sensing of hydrazine. The electrocatalytic performance of the prepared electrode for hydrazine was compared with the bare glassy carbon electrode (GCE) and ZnO GCE using the cyclic voltammetry technique. In the presence of 0.1 mM hydrazine concentration in PBS buffer solution, higher anodic and cathodic currents reveal the ZnO/PTH electrode’s sensitive nature compared to the other electrodes. The oxidation current of 2.5 µA was observed, which was two times more than the ZnO/GCE. Further amperometric studies revealed the electrode’s very sensitive nature by adding different concentrations (0.5 to 48 μM) of hydrazine after regular time intervals. The detection limit was found to be 0.207 μM with a sensitivity of 1.22 μAμM−1cm−2. The highly sensitive nature of the electrode is owed to the synergistic effect of the zinc oxide. Electrochemical sensor can respond more quickly because ofthe rapid electron transfer processes that are made possible by the combination of polythiophene and ZnO nanoparticles. Higher sensitivity is achieved by ZnO nanoparticles high surface-to-volume ratio, which creates a larger area for analyte interaction and improves electron transfer kinetics [122].
8.7. Iron III oxide NPs-CPs
α-Fe2O3 possess good catalytic, low toxic, and eco-friendly properties, making them the material of choice for electrochemical sensors. Adel A. Ismail et al. developed the α-Fe2O3/cross-linked polyaniline- based binary nanocomposite. The synthesized nanocomposite unfolds better electrochemical results in contrast with bare and α-Fe2O3. CVs were recorded in buffer 0.1 M PBS (pH 7.4) at a scan rate of 50 mVs-1 in the presence of bare GCE and α-Fe2O3/CPANI. No oxidation peak was observed for bare GCE, while a visible oxidation peak was observed for α-Fe2O3/CPANI GCE. The exceptional sensitivity of 1.93μAμM−1cm−2, a very low limit of detection (LOD) of 0.153 μM at (S/N=3), and wide-ranging linear hydrazine concentrations from 0.2 μM to 40 μM was confirmed. The significant rise in peak current combined with a decrease in over potential indicates a higher charge transport reaction. As compared to the bare electrode, the results indicate that the nanocomposite material made of iron oxide nanoparticles embedded in a polyaniline matrix has superior electrochemical characteristics. The obvious oxidation peak seen in the CVs obtained with the nanocomposite GCE, which denotes a greater charge transport reaction, serves as its proof. In addition, the nanocomposite displays remarkable sensitivity, a low limit of detection, and a broad linear range of detection for hydrazine. These findings imply that nanocomposite material may function well as a hydrazine sensor. Thus, hydrazine could be sensed efficiently due to iron oxide nanoparticles in the polyaniline matrix [123].
8.8. SrTiO3 NPs-CPs
In 2020, an approach was carried out for the fast and selective detection of hydrazine by M. Faisal et al. Polyaniline and mesoporous Strontium titanate (SrTiO3) nanocomposite was designed and modified on glassy carbon electrode (GCE). The electrochemical performance of the PANI/SrTiO3 was tested against the pure SrTiO3 and bare GCE. Cyclic voltametric response of 5% PANI/SrTiO3 was tested in 0.1 M PBS at 7.2 pH in the presence of 1.0 mM of HZ, revealing a higher oxidation current than bare GCE pure SrTiO3. LSV studied showed the sluggish electron transfer kinetics for bare GCE and pure SrTiO3, while 5% PANI/SrTiO3 disclosed the high affinity of the electron conduction with very low electron transfer resistance. The sensitivity of 0.2438 µAµM-1cm-2 was achieved, while amperometric studies observed a linear detection limit of 0.95 µM. The improved adsorption and diffusion of HZ molecules on the electrode surface owns the synergistic effect of organic and inorganic moieties, thus playing substantial roles in HZ sensing [124]. Therefore an electrode's surface can be altered using SrTiO3 nanoparticles to provide a nanostructured interface that aids in the electrochemical detection of hydrazine. The conducting polymer matrix can allow the nanoparticles to spread uniformly, resulting in the formation of a composite film on the electrode surface. This may give hydrazine molecules a wider surface area on which to interact with the conducting polymer, enhancing the electrochemical reaction (Table 2).
Table 2. CPs-Metal oxide composite electrodes comparison for HZ sensing literature representations

- Ternary Nanocomposites of Conducting Polymers for Hydrazine Detection
The idea of using ternary nanocomposite-based electrochemical sensors for fast and ultra-sensitivity of hydrazine is thriving nowadays. The electrochemical sensing phenomenon of the ternary nanocomposite occurs due to the synergistic amongst the three constituents. Ternary nanocomposites can have better properties than binary nanocomposites, which is one of its advantages. A ternary nanocomposite, for instance, might have increased surface area, better electrical conductivity, or improved catalytic activity. The ability to customize ternary nanocomposites by changing the ratio and content of the three components is another benefit.
The recently prepared CP-based ternary nanocomposite electrochemical sensors for hydrazine detection are discussed here. In a recent attempt, Saeb et al. (2021) prepared TiO2/PANI/Au ternary nanocomposite fabricated on the glassy carbon electrode surface. TiO2 is a wide-bandgap semiconductor that improves sensitivity by expanding the availability of potentially active sites for analyte adsorption. Au nanoparticles have catalytic activity, which increases the sensitivity overall and amplifies the sensing signal. TiO2 nanoparticles were deposited on the GCE surface, followed by the electrodeposition of aniline and gold nanoparticles. The electrochemical performance of ternary nanocomposite for hydrazine was assessed by differential pulse voltammetry. The results showed that linear response of hydrazine concentration vs. current was calculated to be 0.9 × 10-6 M to 1.2 × 10-3 M and the detection limit to 0.5 μM. Other properties such as selectivity, response rate, and reproducibility were studied, and electrodes exhibited good performance. The excellent electrochemical behavior of the electrode towards HZ sensing is attributed to the presence of TiO2 and gold nanoparticles providing high surface area, high charge transfer, and an excellent catalytic effect [125]. G. Kaladevi et al. (2020) prepared silver nanoparticle-modified polyaniline (PANI)/rGO nanocomposite. Reduced Graphene oxide and silver nanoparticles were reacted with the aniline monomer solution to form the ternary nanocomposites. rGo/AgNPs and PANI showed enhanced the peak current response to hydrazine oxidation compared to the bare electrode in 0.1 M PBS (pH=8.0). The diffusion coefficient was determined by chronoamperometry (6.25 × 10−5 cm2/s). The overall synergy of PANI AgNPs/rGO provides a better sensitivity of hydrazine at low concentrations [126]. Rahman et al. have prepared AgNPs-polyaniline tungsten phosphate-based nanocomposite. IV characteristics for hydrazine detection disclosed that the sensor possesses linear response, higher sensitivity of (~12.5 μAcm−2mM−1), and a lower detection limit of (~2.8 nM). It offers more benefits such as constancy, non-hazardous nature, and decent electrochemical activity. The high sensitivity of the synthesized composite allows a fast electron transfer rate. Due to the increased surface area of the nanocomposite, it offers a favourable environment for hydrazine sensing [127]. Yuea et al. (2017) prepared a ternary composite for the hydrazine detection based on nitrogen-doped carbon nanopolyhedra (CNP), Prussian blue (PB), and conductive polymer polypyrrole. PB has outstanding stability and excellent electrochemical characteristics, such as high electrical conductivity. Due to its distinctive capacity for redox reactions, PB is an excellent choice for sensing applications. The electronic characteristics of the carbon lattice are changed by nitrogen doping, which adds more nitrogen atoms to the structure. Moreover, nitrogen increases the number of chemically active sites that can be used, enhancing the sensitivity and selectivity of CNP towards hydrazine molecules. The highly modified electrode demonstrated prompt reaction and sensitivity of 0.22 A.M-1, a dynamic linear range from 7.5×10-7 M to 1.7×103 M, and a detection limit of 2.9×10-7 M, along with significant selectivity and stability. The amperometric response of the sensor was tested by the successive addition of different hydrazine concentrations disclosing its capability for the hydrazine oxidation. The mechanism of hydrazine oxidation is given as follow:

Because of the high electrical conductivity of PPy and the amazing catalytic property given by the PB/CNP/PPy, the electrode modified with PB/CNP/PPy exhibits excellent electro activity towards hydrazine oxidation [128]. Afshari et al. prepared an electrochemical sensor by depositing AgNps on the fluorine-doped tin oxide dispersed in a polyaniline matrix and fabricated on graphitic‑carbon nitride film. The use of nitrogen-based materials is due to their exceptional electrocatalytic properties. Electrochemical deposition of PANI/ graphitic‑carbon nitride (C3N4) film on FTO was achieved at a value of the current intensity of 5 mAcm−2 and different deposition intervals (400 s, 800 s, 1200 s, and 2000 s). The electrochemically active surface area of the electrode expands by increasing the extent of AgNPs and further reveals that up to 1200s, the electrode showed the efficient sensing of hydrazine. The PANI/g-C3N4/AgNPs electrode, in contrast to impure Ag and PANI/g-C3N4 electrodes, shows superior electrochemical detection of HZ. Furthermore, the PANI/ g-C3N4/AgNPs electrode demonstrated a wide linear concentration range of hydrazine (5 to 300 mM) with a detection limit of 300 Mm [129]. Balwinder Kaur et al. used nanocrystalline zeolite to advance the catalytic properties of the CP polymer matrix. The sensing property of copper nanoparticles decorate polyaniline zeolite (CuNPs/PANI-Nano-ZSM) glassy carbon electrode toward hydrazine was studied. The electrocatalytic property was measured using the DPV technique in 0.1M PBS (pH=8.5). Results show that hydrazine was oxidized, indicating a sharp oxidation peak of 595 mV. The oxidation currents increase directly with the increase in HZ concentration, and the linear dynamic range was summed up to be 3nM to 900 μM. HZ was discovered to have a linear calibration in the region of 3 nM to 900 M. The results can be endorsed by the mutual impact delivered by widely distributed CuNPs, conductive PANI, and greater surface area nano zeolite. The easy electron movement was offered by the PANI matrix and nano zeolite [130]. Vellaichamy et al. synthesized ternary nanocomposite based on copper nanoparticles-polyaniline-graphene oxide (CuNPs-PANI-GO) by in situ polymerization. The GO surface was modified by polymer and copper nanoparticles. Intended for carcinogenic hydrazine detection, CV graphs were taken in the 0.1 phosphate buffer solution (PBS) of pH=7.0. The potential is applied from 0.0 to 1.1 V at the scan rate of 50 mV.s−1, whereas increasing the HZ concentration from 10 to 90 µM, the magnitude of oxidation current increases. This is owed to hydrazine’s direct (anodic) electro-oxidation on the CuNPs-PANI-GO electrode surface. To determine the sensitivity, linear range, and detection limit of CuNPs-PANI-GO modified electrode, amperometric studies were done, which are found to be 0.0045, 0.015 µM, and 359.93 µA mM−1.cm−2, respectively, as demonstrated in Figure 6. It was revealed that the ternary nanocomposite of CuNPs-PANI-GO unveils improved electron transfer with the more extraordinary electrochemical performance due to the powerful synergistic effect that owns to the interactions among copper nanoparticles dispersed in the PANI matrix on GO [131].

Figure 6. (a) CuNPs-PANI-GO/GCE graph, (b) CuNPs-PANI-GO/GCE in 0.1 M sol in 50 µM HZ, and (c) CuNPs-PANI-GO/GCE graph at various scan rates. Oxidation current linear relationship with the square root of scan rate [131].
Nitrogen-doped-graphene poly vinyl pyrrolidone / gold nanoparticles (NG-PVP/AuNPs) were prepared for hydrazine oxidation by Saengsookwaow et al. (2016). The use of nitrogen as a heteroatom provides various advantages and improves the electron transfer rate for electrochemical sensing. The CP matrix's electrocatalytic activity and selectivity towards particular analytes may be improved by nitrogen dopants. Graphene and gold nanoparticles can be stabilised and dispersed by the polymer polyvinylpyrrolidone (PVP), which enhances the stability and homogeneity of the composite. In addition, PVP can provide the composite a compatible surface, making it appropriate for sensing applications. The NG-PVP nanocomposite was fabricated on the screen-printed electrode, and the morphological study was done by scanning electron microscopy. SWV was used to study the electrochemical behavior of hydrazine oxidation. Owing to the synergic influence of NG-PVP and AuNPs, the designed electrode showed enhancement in anodic peak ten folds in contrast to the bare screen-printed electrode (SPE). In ideal conditions, high sensitivity of 1.370 μA μM-1cm−2, a wide linear range of 2-300 μM, and a low detection limit of 0.07 μM were acquired for hydrazine [132]. Omar et al. have prepared zinc cobaltite (ZnCo2O4) nanoparticles with a hydrothermal approach and dispersed them in a polyaniline matrix prepared by oxidative chemical polymerization. ZnCo2O4 is a semiconducting nature metal oxide with catalytic activity that aids in the oxidation of HZ. ZnCo2O4 nanoparticles added to the PANI matrix can improve the sensing. The prepared ternary nanocomposite was loaded on the ITO to design a working electrode for hydrazine sensing. Cyclic voltammetry of bare ITO and PANI-ZnCo2O4 were recorded. The bare ITO showed sluggish and irreversible electron transfer. At the same time, PANI-ZnCo2O4 displayed a high anodic current peak due to zinc cobaltite nanoparticles which provide high conductivities, as demonstrated in Figure 7. PANI-ZnCo2O4 nanocomposite was further employed for N2H4 sensor application compared to bare ITO containing 0.3 M NaOH solution with N2H4 concentration changing from 0 to 4 mM at a scan rate of 20 mV/s disclosed in Figure 8. The PANI-ZnCo2O4 showed a high anodic peak current, while bare ITO showed no significant peak showing its slow and sluggish nature. Furthermore, amperometric studies revealed the detection limit for HZ to be 0.2 µM and a linear range of 0.6 mM to 1.05 Mm. The high sensitivity of PANI-ZnCo2O4 nanocomposite is owed to the collective electrocatalytic effect with different redox behaviors of both PANI and ZnCo2O4 [133].

Figure 7. (a) the CV curves of the ZnCo2O4, PANI, and PANI-ZnCo2O4, (b) scan rate effect of PANI-ZnCo2O4, and (c) the CV curves of bare ITO and PANI-ZnCo2O4 in 0.3 M NaOH.

Figure 8. (a) PANI-ZnCo2O4 CV display in 0.3 M NaOH with N2H4 concentration altering from 0 to 4 mM at a scan rate of 20 mV/s and (b) linear relationship between the anodic peak currents versus N2H4 concentrations [133].
Yang et al. have prepared the ternary nanocomposite of Iron oxide/polypyrrole/graphene oxide (Fe3O4/PPy/GO) for the HZ detection. Due to its unique properties, Fe3O4 is the appropriate magnetic iron oxide for sensing applications. Because it has many functional groups and a wide surface area, graphene oxide (GO) is useful because it raises the sensitivity of the composite by offering plenty of sites for analyte adsorption. Additionally, the electrical conductivity of GO is strong, enabling quick electron transfer during the sensing process.Fe3O4/PPy/GO electrochemical activity was checked compared to bare GCE by CV in PBS sol of 7.0 PH. The CV results revealed that Fe3O4/PPy/GO showed a higher oxidation current while bare GCE and GO/GCE showed no significant oxidation current in 0.5 mM HZ. The amperometric response showed the current vs. time graph, revealing that the oxidation peak increases by consecutive addition of HZ. The linear detection ranges of 5.0 μM to 1.275 mM were found. The detection limit for HZ was 1.4 μM. The improved electrocatalytic activity of Fe3O4/PPy/GO was attributed to the combined influence of nanoparticles and polypyrrole possessing higher surface area, good electrocatalytic properties, and high conductivities [134]. Comparison of various ternary composite results for HZ sensing are given in Table 3.
Table 3. Literature comparison of various CPs ternary composite electrode for HZ sensing

- Perspective Outcome & Conclusion
The research and development of electrochemical sensing systems can be advanced through the introduction of nanomaterials into conducting polymers. These sensors can detect toxic hydrazine quickly, selectively, and sensitively by taking advantage of the superior characteristics of nanoscale materials, such as improved electrode kinetics, active electron transfer, and improved catalytic activity. This opens the door for improved safety and monitoring capabilities across a variety of fields. A conducting polymer nanocomposite for hydrazine sensing should take into account a number of crucial variables, including, sensing mechanism, sensitivity and selectivity. Conducting polymers and nanocomposite components should be selected based on their tendency to interact with hydrazine and to experience quantifiable changes in their electrical properties when exposed to hydrazine. To provide precise and dependable sensing performance, the sensing mechanism should be thoroughly understood. One of the most important factors is how sensitive the conducting polymer nanocomposite is to hydrazine. In the hydrazine presence at low concentrations, the nanocomposite should show a considerable change in its electrical properties, enabling precise and reliable detection. To prevent false positives or false negatives, the conducting polymer nanocomposite selectivity towards hydrazine is crucial. In spite of other potentially interfering species that are frequently present in the sample matrix, the nanocomposite ought to be able to detect hydrazine with high specificity.
Abbreviations
CPs: Conducting polymers
CNPS: Carbon nanoparticles
CV: Cyclic Voltammetry
DPV: Differential Pulse Voltammetry
FTIR: Fourier Transform Infrared
GCE: Glassy Carbon Electrode
HZ: Hydrazine (N2H4)
LOD: Limit of detection
LSPR: Localized Surface Plasmon Resonance Spectroscopy
MNPS: Metal Nanoparticles
NGPVP: Nitrogen-Doped Graphene Polyvinyl Pyrrolidine
PANI: Polyaniline
SERS: Surface Enhanced Raman Spectroscopy
TEM: Transmission electron microscopy
SEM: Scanning electron microscopy
PPy: Polypyrrole
RGO: Reduced Graphene Oxide
ZIF: Zeolitic Imidazole Framework
ZSM: Zeolite
PEDOT: Poly-3, 4-Ethylenedioxythiophene
ITO: Indium Tin Oxide
Mm: Milli mole
µM: Micro mole
Sol: Solution
AuNPs: Gold nanoparticles
PdNPs: Palladium Nanoparticles
ZnO: Zinc Oxide
SS: Stainless steel
SrTiO3: Strontium titanate
C3N4: Graphitic‑carbon nitride
FTO: Fluorine Tin oxide
ZnCo2O4: Zinc cobaltite
Fe3O4: Iron Oxide
NaOH: Sodium hydroxide
CuNPs: Copper Nanoparticles
nM: Nano molar
PKa: Acid dissociation constant
LBL: Layer by layer
AgNPs: Silver Nanoparticles
WHO: World Health Organization
NTs: Nanotubes
SPE: Screen Printed Electrode
1D: One Dimension
2D: Two Dimension
WE: Working Electrode
EIS: Electrochemical Impedance Spectroscopy
SWV: Square Wave Voltammetry
µA: Micro Ampere
Acknowledgment
Authors are thankful to the Hazara University, Mansehra, Pakistan and National center for physics, Islamabad, Pakistan for providing opportunity. We are indebted to for their unwavering support, which has significantly contributed to the successful completion of this review article.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Orcid:
Muhammad Nouman Siddique Awan: http://orcid.org/0000-0003-2520-1346
Amina Othmani: http://orcid.org/0000-0003-1787-9683
Humaira Razzaq: https://orcid.org/0000-0002-4650-8729
Obaid Ur Rahman Abid: http://orcid.org/0000-0002-3553-6089
Citation: M. N. S. Awan*, H. Razzaq, O. U. Rahman Abid, S. Qaisar, Recent Advances in Electroanalysis of Hydrazine by Conducting Polymers Nanocomposites: A Review. J. Chem. Rev., 2023, 5(3), 311-340.


