Document Type : Review Article
Authors
Institute of Chemical Sciences, University of Swat, Swat, Pakistan
Abstract
Magnetic nanoparticles (MNPs) have emerged on the central stage in material sciences with diverse applications especially in biomedical and environmental fields. This review focuses on the recent development of nickel and cobalt magnetic nanoparticles. Various methods of synthesis, composition, characterization, and applications have been discussed in this article. The main aim of the review is to highlight that not only iron nanoparticles show magnetic properties and applications, but also nickel and cobalt nanoparticles exhibit such behaviour in different types of smart materials. The findings of this study are that similar methods of synthesis and characterization can be equally applied to Ni and Co MNPs just like Iron MNPs.
Graphical Abstract
Keywords
Main Subjects
- Introduction
agnetic nanoparticles (MNPs) have emerged as highly versatile materials with a remarkable array of applications attracting widespread attention and research efforts within the scientific community over the past two decades [1,2]. These nanoparticles exhibit a unique set of properties, including precise size control, well-defined crystalline structures, and exceptional stability in various solvents making them indispensable in an ever-expanding range of scientific and industrial domains [3,4]. This comprehensive review endeavors to provide an in-depth exploration of the synthesis, characterization, and diverse applications of nickel and cobalt-based MNPs shedding light on the significant strides made in recent years towards harnessing the immense potential of these nanoparticles. MNPs play a pivotal role in a multitude of applications spanning fields such as biomedicine, environmental remediation, energy storage, data storage, catalysis, and magnetic resonance imaging (MRI). Their unique combination of magnetic properties coupled with their tunable sizes and shapes has rendered them indispensable in addressing contemporary scientific and technological challenges. By meticulously examining the synthesis methodologies and recent advances, this review aims to offer valuable insights into the utilization of MNPs as a central building block for an array of innovative applications [5-8].
A central theme in this review revolves around the various methods employed for the MNPs synthesis. One widely adopted approach is thermal decomposition. This technique involves the controlled decomposition of precursor in the presence of organic surfactants at elevated temperatures. The resultant MNPs are characterized by precise size control, uniform shapes (often spherical) and remarkable resistance to oxidation. The magnetic characteristics of these MNPs are intricately linked to their individual sizes allowing for fine-tuning of their properties through manipulation of synthesis parameters [9-12]. Another prominent method is chemical reduction known for its simplicity, environmental friendliness, and scalability. Chemical reduction involves the reduction of metallic salts using suitable reducing agents while stabilizing ionic complexes through capping agents. This method has been pivotal in producing fine particles of ferromagnetic transition metals such as nickel and cobalt in powdered form which display excellent dispersion and stability catering to a wide spectrum of applications [13-17]. The wet chemical method, on the other hand, leverages chemical reactions in liquid solutions for nanoparticle synthesis. This versatile method enables the use of various precursors and allows the production of different shapes and morphologies of MNPs, expanding their applicability. Recent advancements in this technique have further extended its potential for tailoring MNPs to meet specific requirements [18-21]. In contrast, the sol-gel method offers an environmentally conscious approach using readily available starting materials. It ensures even blending well-defined crystalline properties and precise size distribution of the nanoparticles. This approach exemplifies an economical route for the synthesis of substantial quantities of MNPs while maintaining precise size control. The solution-phase method provides a straightforward means of achieving uniformly distributed small-sized MNPs. This method relies on surfactants to prevent nanoparticle aggregation, ensuring uniform dispersion. It has been successfully employed in the synthesis of nickel nanoparticles, with prominent applications [22-24].
The MNPs potential transcends their synthesis methodologies, extending to a multitude of applications. In biomedicine, MNPs are utilized for targeted drug delivery, hyperthermia therapy and as contrast agents in MRI. They have also made significant contributions to environmental remediation where they are employed in wastewater treatment and pollutant removal [25].
In the realm of energy storage, MNPs play a crucial role in the development of advanced batteries and supercapacitors enhancing energy density and charge-discharge efficiency. Data storage benefits from MNPs in the form of high-density magnetic storage media while catalysis exploits their surface properties for enhanced catalytic performance [26]. As we delve into the multifaceted applications of nickel and cobalt-based MNPs, it becomes apparent that these nanoparticles are pivotal in addressing some of the most pressing challenges in science and technology today. Their unique magnetic properties coupled with the ability to tailor their size and shape make them indispensable building blocks for innovation across a spectrum of fields [27].
- Main Synthesis Methods for Nickel and Cobalt Magnetic Nanoparticles (MNPs)
Main synthesis of nickel and cobalt MNPs is shown in Figure 1.
Figure 1. Main synthesis of nickel and cobalt MNPs
2.1. Thermal decomposition
The approach used in creating these MNPs results in excellent crystalline properties, precise size control, clearly defined shapes such as spherical, monodisperse, and uniformed with ability of self-assembly. The MNPs produced exhibit stability in hydrocarbon solvents, displaying resistance to oxidation from air exposure. The magnetic characteristics of the nanoparticles rely on their individual sizes [28-30]. The decomposition of precursors occurs in the presence of organic surfactants mainly oleylamine, trioctylphosphine, and triphenylphosphine. In this method, different precursors are used to produce monodisperse MNPs under extreme temperature (240-245 oC) such as Nickel(II) acetylacetonate; Ni (acac)2 [31], Nickel (aceto)2-oleylamine [32], Cobalt octacarbonyl; Co2(CO)8 [30], Cobalt nitrate hexahydrate, Co(NO3)2·6H2O [33], and Cobalt bis(salicylidene) [29]. Control of the crystalline phase is influenced significantly by factors such as reaction temperature, duration, heating rate, and the type of solvent used. Varying these reaction parameters can lead to the formation of either face-centered cubic (FCC) or hexagonal close-packed (HCP) magnetic nanoparticles [34]. In comparison to co-precipitation, the thermal decomposition technique proves to be more advantageous in the production of smaller-sized magnetic nanoparticles [35]. The magnetic characteristics of organized arrays of cobalt nanoparticles exhibit enhancements when contrasted with cobalt nanoparticle powder compressed within an enclosure or mixed with wax) [30]. Both experimental and theoretical analyses are conducted to explore the magnetic characteristics of the MNPs across varying particle sizes [35]. The as prepared MNPs size ranges from 2-114 nm in diameter (Table 1) [29,36].
Table 1. Methods of synthesis used for synthesis of Ni and Co MNPs
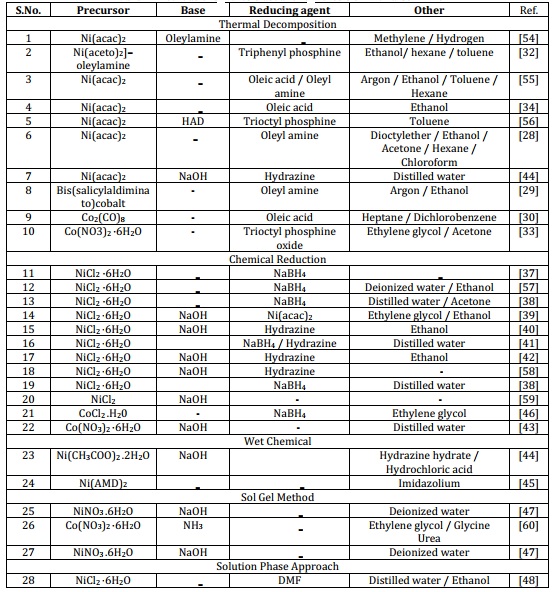
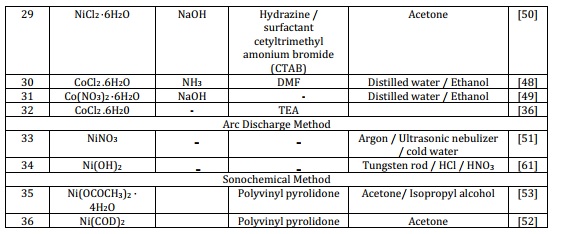
2.2. Chemical reduction
The chemical reduction process comprises three fundamental phases: the reduction of metallic salts using reducing agents, ionic complexes stabilization, and size regulation facilitated by the capping agent. This approach is straightforward, environmentally friendly, and yields remarkable product quantities without the need for separation methods like column chromatography. Chemical processes possess a notable benefit in generating materials in powder format. Consequently, chemical reduction has evolved into an essential method for manufacturing fine particles of ferromagnetic transition metals (such as nickel and cobalt) and their alloys in powdered state [37]. Chemical reduction can be employed to create fine nickel particles by utilizing nickel salt like nickel chloride hexahydrate in combination with a reducing agent such as sodium borohydride. Ni nanoparticles at the nanoscale achieved through the reduction process using borohydride [38]. Nanocrystalline nickel (Ni) nanoparticles with a face-centered cubic structure are synthesized at a temperature of 60 °C. This process involves the utilization of NiCl2 as a precursor, hydrazine hydrate as the reducing agent, and ethylene glycol as the capping agent [38-42]. This technique leads to the synthesis of nickel nanoparticles, initiated by dissolving nickel chloride hexahydrate in water. Subsequently, hydrazine hydrate is introduced and the process is supplemented with the addition of NaOH to uphold alkaline conditions [42]. The production of cobalt magnetic nanoparticles can be achieved through a chemical reduction approach involving salts within alkali solutions. The synthesis of Co nanoparticles is initiated by the reduction of Co(NO3)2, followed by a subsequent high-temperature hydrogen reduction lasting 30 to 150 minutes. The resulting samples are subject to analysis through TGA and XRD techniques (Table 1) [43].
2.3. Wet chemical method
The approach involving wet chemical method is another commonly used technique for synthesizing magnetic nanoparticles. It involves the use of chemical reactions in a liquid solution to produce the nanoparticles. Various precursors can be used depending on the desired properties of the nanoparticles. Some common precursors include metal salts, metal oxides, and organic compounds. These precursors are typically dissolved in a solvent such as water or an organic solvent and then subjected to various chemical reactions, such as precipitation, hydrolysis, or reduction to form the magnetic nanoparticles [44]. The selection of precursor materials and manipulation of reaction parameters significantly impact the dimensions, configurations, and magnetic attributes of the nanoparticles [45]. Under alkaline conditions, nickel nanoparticles are produced through the reduction of a nickel-complex that arises from a mixture of nickel acetate and hydrazine solution. In recent years, various shapes and morphologies of nickel and cobalt magnetic nanoparticles have been successfully synthesized using this technique. Examples include nanotubes, nanorods, hollow spheres, nanobelts, nanoprisms, and hexagonal flakes (Table 1) [44,46].
2.4. Sol-gel method
This technique is environmentally conscious utilizing cost-effective chemicals and saving time thus presenting a straightforward and economical pathway due to the employment of readily available and limited starting materials. The sol-gel method offers various advantages including even blending, well-defined crystalline properties, and a consistent precise size distribution of the resultant nanoparticles. The sol-gel technique enables the production of significant quantities of MNPs while maintaining size control and precise shaping [47]. NiO nanoparticles are synthesized via a sol-gel method, where nickel chloride hexahydrate is employed as the precursor and polyvinylpyrolidone serves as the capping agent. The sol-gel method was employed to successfully synthesize NiO magnetic nanoparticles with nickel nitrate hexahydrate and sodium hydroxide as the key constituents. The findings revealed the generation of pure NiO nanoparticles without any trace of impurities (Table 1) [47].
2.5. Solution phase approach
The synthesis of magnetic nanoparticles using a solution-phase method is a straightforward means to achieve the production of uniformly distributed, small-sized MNPs. This method involves using surfactants to prevent the nanoparticles from aggregating. One specific example is the synthesis of nickel nanoparticles using dimethylformamide both as the reductant and solvent [48]. This study represents the primary instance of employing DMF for the fabrication of nickel nanoparticles. Furthermore, this technique can be further applied to generate cobalt magnetic nanoparticles. In this case, cobalt acetate tetra hydrate serves as the precursor, while sodium borohydride acts as the reducing agent. Employing various surfactants can influence the magnetic characteristics of cobalt MNPs. Utilizing oleic acid as a surfactant yields ferromagnetic cobalt nanoparticles exhibiting a room temperature coercivity of 583. Interestingly, the incorporation of both oleic acid and trioctylphosphine surfactants in the process results in a noteworthy decrease in coercivity to 360.6 Oe [49]. Moreover, his solution-based approach can be employed to craft various nanoscale metals and intricate structures (Table 1) [48,50].
2.6. Arc discharged method
A controlled synthetic approach was formulated to produce nickel nanoparticles through the utilization of an arc discharge technique coupled with an ultrasonic nebulizer. This method, characterized by its cost-effectiveness and environmental friendliness, offers an efficient means of generating magnetic nanoparticles of nickel and cobalt. These nanoparticles exhibit a narrow size distribution, high purity, and spherical shape. Spherical fine Nickel magnetic nanoparticles are prepared through this method. The magnetic characteristics of the produced nickel magnetic nanoparticles are significantly influenced by their size. As the particle size decreases below a critical threshold, a distinctive superparamagnetic behavior becomes evident (Table 1) [51]. This fabrication technique is widely employed in the creation of magnetic nanoparticles for diverse applications, including magnetic data storage, biomedical imaging, and catalytic processes.
2.7. Sonochemical method
The sonochemical method offers Benefits like swift fabrication and homogeneous particle size distribution and having the capability of controlling the size and shape of the magnetic nanoparticles. The sonochemical technique for the preparation of nickel and cobalt magnetic nanoparticles involves the use of ultrasonic waves to induce cavitation in a liquid medium containing metal precursors [52]. Easily achievable via a sonochemical synthesis within a PVP-assisted reaction setup, nickel nanoparticles can be obtained with manageable size and structure. These findings provide a direct route to modulate the magnetic attributes of nickel nanoparticles by regulating their self-organization over time (Table 1) [53]. This method offers the capability to convert cobalt into its zero-valent state using calcium instead of hydrogen. A notable benefit of employing this method is its capacity to recycle oxidized nanoparticles back into cobalt nanoparticles, all without necessitating chemical solvents or involving complex, multi-step procedures [26]. In addition, the sonochemical method offers a fast and efficient way to produce magnetic nanoparticles with excellent dispersibility and stability.
- Composition of Magnetic Nickel and Cobalt Nanoparticles
3.1. Oxides
Metal oxide magnetic nanoparticles (MNPs) have garnered significant interest in recent times due to their magnetic properties and inherent chemical stability. These attributes hold great promise for potential applications in the fields of magnetic separation and biomedicine. They are synthesized through a straightforward procedure primarily relying on nickel and cobalt complexes in alkaline environments [62].
By regulating the solvent and employing oleylamine as a surfactant, the intended particle size can be achieved. Oleylamine, serving as a capping agent, limits particle growth during this phase of the procedure. Utilizing oleylamine as a surfactant for adjusting the energy of the particle surface, diverse morphologies of nickel and cobalt oxides can be achieved. This approach facilitates directed growth, leading to formations such as octahedral Ni3O4 nanoparticles and Co3O4 nanoprisms [63, 64].
Particle growth along a specific direction can be managed by adjusting the surfactant quantity. Furthermore, through thermal annealing, nickel oxide can be converted into cobalt oxide. Analogous approaches were taken to synthesize cobalt oxides. In the presence of PVP, a hydrothermal procedure led to the formation of nanoscale platelets (NPLs) composed of cobalt (II) hydroxide [Co (OH)2] from cobalt nitrate [Co (NO3)3]. This process could potentially give rise to cobalt oxide MNPs [65]. Examination of the magnetic characteristics of the samples through the VSM technique reveals that the NiO nanoparticles (post-calcination) exhibit superparamagnetic properties [66].
3.2. Carbides
In spite of their notable magnetic attributes and stability, nickel and cobalt carbides (Ni5C2, Ni3C, and Co2C) have received limited attention due to the difficulties encountered in their synthesis, particularly in achieving precise control over size and morphology. Ni5C2 magnetic nanoparticles were synthesized through the decomposition of Ni (CO)5 in the presence of octadecylamine [67].
Highly crystalline nickel magnetic nanoparticles were subjected to carbonization to create Ni5C2. In an effort to modulate surface energy, bromide was introduced during the carbide MNPs synthesis, though the underlying mechanism remained ambiguous. The resulting Ni5C2 MNPs, with a diameter of 20 nm, exhibited an amorphous outer layer. A synthetic chemical pathway was devised, leading to the formation of Ni carbide MNPs with distinct crystalline structures [68]. The nickel carbides display subtle ferromagnetic attributes, suggesting that diverse synthetic pathways for their creation might yield various types. This is particularly evident when halide ions are introduced, as they may influence the absorption of carbon content through selective means.
- Characterization of Nickel and Cobalt Magnetic Nanoparticles
The nickel and cobalt magnetic nanoparticles (MNPs) are examined using various tools and methods to ascertain their dimensions, form, and structure (Figure 2) [69]. A few of the devices employed for their analysis comprise atomic force microscopy (AFM), (FT-IR) spectroscopy, ultraviolet spectrometry, (TEM) transmission electron microscopy, and (MS) Mossbauer spectroscopy. The zeta potential is employed to assess the stability of the nickel and cobalt MNPs [51,70].

Figure 2. Different characterization parameters of Ni/Co MNPs
4.1. Size, shape and morphology
Utilizing scanning electron microscopy (SEM) provides numerous benefits in determining the dimensions, form and structure of the nanoparticles. The mean size is computed based on SEM analysis. XRD is employed for attaining a uniform configuration of nickel and cobalt magnetic nanoparticles (MNPs) [9,51]. Method such as transmission electron microscopy (TEM)/high resolution TEM(HRTEM) and atomic force microscopy (AFM), SEM/filed - emission scanning electron microscopy (FESEM) can gauge the surface structure of nickel and cobalt MNPs. The image obtained through this device furnish insights into their form and dimensions, which enables the calculation of their diameter [46, 71]. The AFM methodology is utilized to gauge step height, surface roughness, and particle distribution. TEM imparts valuable information about composition, structure, and dimensions of these MNPs. SEM on the other hand, furnishes data about surface features and sample composition. Transmission electron microscopy proves exceedingly beneficial to attain an evaluation of crystallinity, aggregation state of MNPs, lattice spacing and electron phase displacement. The distinct peaks evident in XRD are amenable to size computation for MNPs, a process facilitated the Scherer equation. Conversely, amorphous MNPs yield broader peaks that complicate size determination [34,60,70]. The XRD utilization serves to elucidate the crystalline nature of nickel and cobalt MNPs. Both XRD and TEM can determine both the average particle size and its distribution.
4.2. Elemental composition
Various tools such as (TEM) transmission electron microscopy, (XPS) X-ray photoelectron microscopy, and (SEM) scanning electron microscopy (SEM), have the capability to establish the elemental constitution and surface structure [25, 72]. Determination of the elemental makeup in nickel and cobalt MNPs is also facilitated by atomic absorption spectrophotometry (AAS). The elemental composition of the synthesized materials is established through the utilization of energy-dispersive X-ray spectroscopy (EDX), a micro-analytical technique often employed in conjunction with SEM [73-75]. The magnitude of the peak in the EDX spectrum provides insights into the elements’ concentration within the sample [47,75]. Similarly, (XRF) X-ray fluorescence is employed to ascertain the elemental structure of these MNPs. Preparing samples for XRF analysis is straightforward, quick, and secure, especially when contrasted with alternative approaches. It has the ability to identify elements quantities in minute as 100 ppb (parts per billion). Furthermore, X-ray diffraction (XRD) can also serve to identify oxides or hydroxides.
4.3. Type of bonding and structure
The arrangement and connection qualities of magnetic nanoparticles are ascertained using various methods. The methods employed are Fourier transform infrared, X-ray absorption spectroscopy), and thermos-gravimetric analysis, X-ray photoelectron microscopy and Raman spectroscopy. X-ray photoelectron microscopy is applicable for the exterior arrangement of MNPs, which furnishes details on the composition and speciation of components [43,76]. Fourier transform infrared and x-ray photoelectron microscopy facilitate the identification of the interaction between organic and inorganic materials, particle binding energy and oxidation state. Fourier transform infrared spectroscopy additionally proves advantageous in understanding the functional clusters of organic molecules. The Raman Spectroscopic method is performed to reveal the compound’s structure and lattice arrangement. The Thermo-Gravimetric analysis technique is utilized to assess the binding effectiveness on the particle surface by providing information about coating formation, particularly with regards to surfactants and polymers. The X-ray absorption spectroscopy provides valuable insights into oxidation states and fundamental components of electronic configuration [64, 77].
4.4. Magnetism
The magnetic properties of nickel and cobalt magnetic nanoparticles rely on their construction through various synthetic pathways. The size of Ni MNPs varies from the nano- to micro-scales, exhibiting superparamagnetic properties. When expose to an external magnetic field, these MNPs display magnetic responsiveness and have the capability to interact with the ambient magnetic fields [45,78]. Nonetheless, when an external magnetic field is absent, they do not exhibit any magnetism. The magnetic properties of nickel and cobalt MNPs are evaluated using various methodologies, including VSM and SQUID [51,79]. For the overall measurement of magnetization magnetometry are employed. The Superconducting quantum interference device proves especially useful in analyzing samples in diverse states, encompassing thin films, powders, crystals, liquids, and gases [80]. Both Superconducting quantum interference device and Vibrating sample magnetometry techniques can ascertain magnetic saturation and residual magnetization while maintaining a constant external magnetic field [55,81]. Another reliable technique for assessing the magnetic characteristics of nickel and cobalt MNPs is physical property measurement system (PPMS) [39]. The Vibrating sample magnetometer can assess magnetization of Magnetic nanoparticles typically when exposed to an external magnetic field ranging from -3 to 3T. It is also useful for evaluating the shells impact on magnetization saturation. The physical property measurement system is dependable approach for determining the magnetic characteristics and the MNPs behaviour. This setup is devised for the measurement of the relationship between magnetization, magnetic field, and temperature in samples of MNPs [82-84].
- Applications of Nickel and Cobalt Magnetic Nanoparticles
The MNPs in the previous ten years have garnered significant interest due to their encouraging outcomes across diverse domains. MNPs possessing superparamagnetic characteristics, distinct dimensions, configuration, elevated surface area and volume proportion, and biocompatibility enhance potential of their utilization. As a result of these attributes, it has drawn the attention of numerous researchers from various disciplines. Within this overview, we have condensed the implementations of MNPs in established domains like catalysis, medical science, and magnetic traits, as well as the deterioration of dyes. A summarized outline of MNPs potential uses within these areas is given in Figure 3 while concise delineation is furnished below.
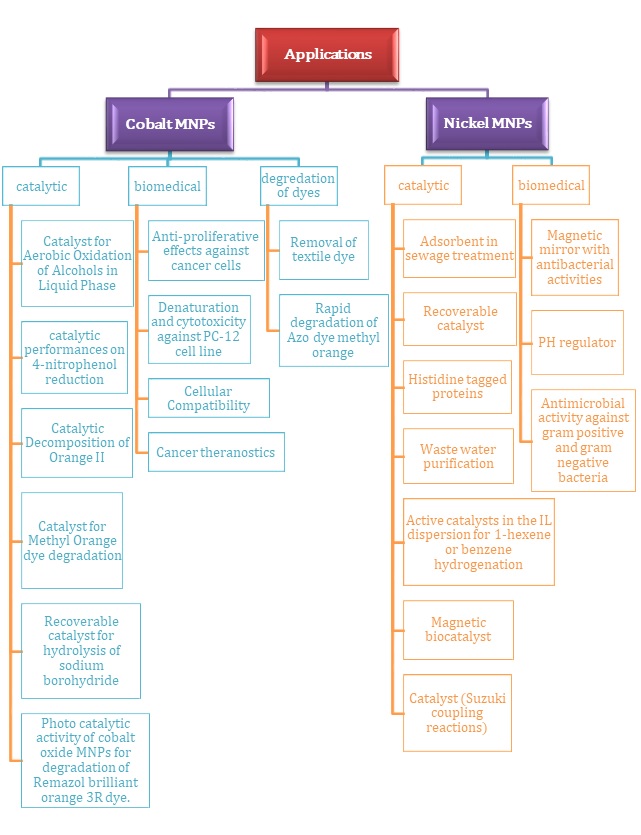
Figure 3. Applications of Ni and CO MNPs
5.1. Catalysis
So far, numerous catalytic process and configurations have been developed
for converting starting materials into final products. One drawback of homogeneous catalysts is the challenge of isolating them from the chemical processes. In recent times, the limitations of heterogeneous catalysis have been alleviated and reduced through the utilization of catalysts supported by magnetic nanoparticles. The Magnetic NPs possess the capability to offer an extensive exterior area to uphold effective sites for effortless conversion of starting material into products, simultaneously merging the benefits of high dispersion and reactivity in the separation of these catalysts [74]. Nickel and cobalt MNPs can be commonly employed as a catalyst such as oxidation under aerobic conditions of alcohols, reductive hydrogenation and Suzuki coupling reactions. Catalytic hydrogenation of alkene with Ni Magnetic nanoparticle changing a double bond from C=C to a single bond C-C [5,85-86]. Due to their magnetic and catalytic attributes, these nanoparticles find extensive utility across diverse domains. Large-sized monodisperse nickel nanoparticles were effectively employed for the purification of proteins carrying histidine tags (Table 2) [28]. Nickel and cobalt magnetic nanoparticles can serve as a promising adsorbent in industrial wastewater treatment, particularly for effluents containing CR. The experimental findings indicate that the Ni nanoparticles produced in this manner could serve as a viable adsorbent in sewage treatment applications [22, 57]. Magnetic nanoparticles of nickel and cobalt have been engineered to create magnetic biocatalysts, enhancing recovery rates, reusability, and catalytic efficacy in the lipase-Ni system for both hydrolytic and synthetic reactions. This approach offers a straightforward and cost-effective method to generate effective and reusable magnetic biocatalysts, showcasing potential applicability on an industrial scale [87-88]. In recent times, the photochemical catalytic system has emerged as an effective and dependable technique for the degradation of pollutants using natural light. Within this framework, sunlight acts as an external stimulus to activate the system, generating free radicals that interact with pollutants and initiate degradation processes (Table 2) [79,89].
Table 2. Applications of Ni and Co MNPs
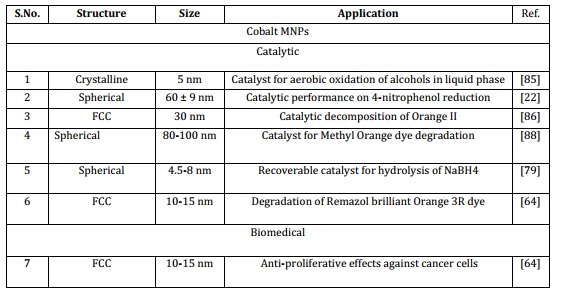
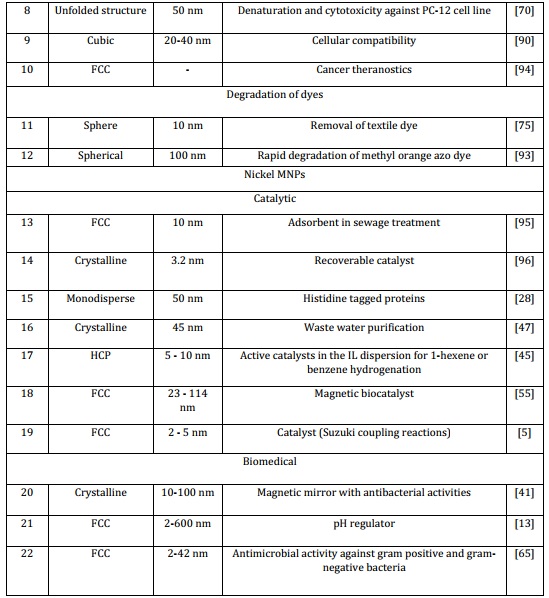
5.2. Biomedical
In recent times, magnetic nanoparticles (MNPs) have gained significant prominence in various biological applications due to their diverse physicochemical properties, convenient synthesis, stability, and biocompatibility. MNPs can effectively interact with external magnetic fields and influence the surrounding magnetic environment, thus enhancing magnetic resonance imaging (MRI). The application of an external magnetic field induces different forces and torques on dipoles, leading to translation, rotation, and energy dissipation. These phenomena find extensive utility in numerous fields such as cell separation and biomarker detection, targeted drug delivery through magnetic means, manipulation of cell surface receptors via magneto-mechanical interactions, biomedical imaging, bacterial theranostics, controlled drug release activation, and hyperthermia treatment. The MNPs composition can vary, resulting in distinct physical and magnetic properties tailored to specific applications. However, in the realm of biomedical research, a crucial consideration is their potential biocompatibility and toxicity [74]. Cobalt and cobalt oxide magnetic nanoparticles (MNPs) have found various biomedical applications, including acting as enhancers for magnetic resonance imaging (MRI), demonstrating anti-proliferative effects on cancer cells, and enabling hyperthermia treatment. These MNPs have proven valuable in biomedical sensing, contrast-enhanced treatment for malignant cells, and as carriers for targeted drug delivery in cancer therapies. Moreover, they have exhibited notable antimicrobial efficacy against both Gram-positive and Gram-negative bacteria [64,72,90]. Magnetic nickel and cobalt nanoparticles are used as magnetic mirror ,antibacterial activities such as (S. aureus and E. coli bacteria) and magnetic properties [41]. These MNPs can also be applied in surgery and laser tools due to as the reported properties present in this NMNPs [13,91]. It has been documented that functionally charged nickel nanoparticles (NiNPs) have the capability to enhance the permeability of cellular membranes, thereby facilitating the uptake of target molecules by cancer cells. These findings indicate a potential mechanism by which Ni NPs could selectively target the cytotoxicity of leukemia cancer cells, implying their potential applications in various biomedical and clinical contexts [65]. Consequently, utilizing smaller NiO nanoparticles can lead to a more efficient application as an antimicrobial agent (Table 2) [65,70].
5.3. Magnetic properties and degradation of dyes
Magnetic metallic nickel and cobalt nanoparticles offer strong potential for dye adsorption. The elimination of dyes from wastewater is of paramount importance due to their significant presence as pollutants. Organic dyes in wastewater degrade water quality and can have detrimental effects on human health. A majority of these organic dyes are toxic, mutagenic, and carcinogenic. Utilizing composites of nickel and cobalt nanoparticles for the extraction of dyes from aqueous solutions holds great promise [75]. Magnetic nanoparticles of cobalt and nickel are employed for the eradication of Remazol golden yellow RNL (RGY) from both aqueous solutions and textile wastewater. In the case of textile wastewater, cobalt and nickel nanoparticles were employed. The findings demonstrated substantial decolonization, reaching an impressive 88 %. However, the reduction in chemical oxygen demand was only around 32 %, underscoring the efficacy of cobalt and nickel nanoparticles in eliminating organic dyes from aqueous solutions [75,92]. These nanoparticles find application in the separation and purification of His-tagged proteins from complex mixtures, such as cell lysates. Lee et al. have documented the utilization of nickel nanoparticles immobilized on activated carbon, introducing a novel adsorbent for the individual and concurrent adsorption of methylene blue and safranin-O [92]. Genhua Zhang documented the production of surfactant-free nickel nanoparticles, which were subsequently applied to eliminate Congo red, an Azo dye commonly found in industrial wastewater (Table 2) [57,93].
Conclusion
To sum up, we have summarized the recent development of Nickel and Cobalt magnetic nanoparticles summarizing various methods of synthesis, characterization, composition, and applications. There are seven main methods of synthesis with each method equally useful depending on the required application. The composition of these magnetic nanoparticles can be varied with different types of substrates used. Various characterizations can be used that are commonly available for nanoparticles. The main applications of these magnetic nanoparticles are in the field of biomedical and catalysis. The ease of synthesis, commonly available starting materials, and diverse applications of these Ni and Co magnetic nanoparticles has opened new opportunity in the field of nanotechnology.
Acknowledgements
We are sincerely grateful to the University of Swat for support of this work.
Orcid:
Muhammad Omer: https://orcid.org/0000-0002-6453-1906
Ihsan Ullah: https://orcid.org/0000-0002-7666-6197
Citation: M. Khan, S. Khan, M. Omer, M. Sohail, I. Ullah*, Nickel and Cobalt Magnetic Nanoparticles (MNPs): Synthesis, Characterization, and Applications. J. Chem. Rev., 2024, 6(1), 94-114.