Reviewers
Authors
1 Institute of Chemistry, School of Physical Sciences, University of Chinese Academy of Sciences.
2 Department of Physics, Faculty of Physical Sciences, University of Calabar, Calabar, Nigeria
3 National Center for Nanoscience and Technology (NCNST)
4 Department of Chemistry, Faculty of Physical Sciences, Modibbo Adama University of Technology, Nigeria
Abstract
Conducting polymers (CPs) have been gathering a great interest in academia and industry by providing the opportunity of combining the electrical properties of a semiconductor and metals with the traditional advantages of conventional polymers such as easy and low cost preparation and fabrication. In this review we examined the conducting polymers-based composites for supercapacitor and batteries, such as conducting polymer-based binary, ternary, and quaternary composites. For their applications in energy storage field, we critically review the development of their applications and the general design rules for energy storage devices including supercapacitors, lithium and other -ions batteries, and their current limitations and future potential to advance energy storage technologies. It is expected that this review will help to improve the knowledge about this conducting polymer and consequently lead to new research fields.
Graphical Abstract
Keywords
1. introduction
Polymers are familiar to everyone, their familiarity and wide spread use has been due to the advantageous rang of controllable mechanical and viscoelastic properties, where electrical behaviour has been important. Although the major property of polymers has been their high insulating capabilities, except for the special case of tribolelectricity use in electrophotography, but in recent years, it has become increasingly recognise that in the vast class of existing polymers, the range of electrical behaviour is remarkably wide. However, it is only very recent that comprehensive and analytical studies has aimed at understanding the properties and utilization of these polymers. An important characteristics of polymers is the possibility of potential control and modification of properties by using creative chemicals and synthetic concepts resulting from the high level of freedom in carbon-based chemistry [1].
Conducting polymers are new class of materials whose conducting properties were first discoveredin 1977 [2]. Such materials demonstrate remarkable optical and electrical properties which were formerly found only in inorganic systems. Electronics properties of conducting polymers differs from all the well-known inorganic crystalline semiconductors like silicon in two major areas such as long range order and their nature which is molecular [3]. For a polymer to become conducting, the polymer must contain overlapping -molecular orbitals and a high degree of -bond conjugation. This comprehensive π-conjugated system of the conducting polymers has irregular single and double bonds all along the polymer chain (Fig. 1) [4]. When the highly conjugated polymer is in its neutral state, it is essentially an insulating material. It is only upon removal of a -bond electron from the conjugated polymer backbone to form a radial cation defect (called a polaron) that the insulating polymer becomes conductive. Removal of -bond electron causes the remaining electrons in the -orbitals to become delocalized along the length of the conjugated polymer backbone, thereby enabling free movement of the polymer along the chain. The formation of the positive charge polymer backbone is accompanied by the incorporation of a dopant anion which has the effect of electrostatically balancing the positive charges. With the discovery of electrically conducting polyacetylene in 1977 by Shirakawa, MacDiarmid, and Heeger by the partial oxidation of polyacetylene films with gaseous bromine or iodine resulted in a dramatic increase in the electrical conductivity of the polymer with values in the metallic region. Although polyacetylene is the most characterised and conductive (up to 105 S/cm) conducting polymer, its poor environmental stability has limited its commercial potential [5]. The 2000 Nobel Prize for chemistry was consequently awarded to the scientist who discovered the electrically conducting polyacetylene.

Figure 1. π-Conjugated system of conducting polymers
Conducting polymers have received enormous interest over the last decade resulting to an explosion of publications. The research in this area has provided the fundamental understanding of the chemistry, physics and material science of these materials and has supported the industrial grwoth of conducting polymer products [6, 7-12]. Several kinds of CPs have been produced and are widely in use due to reasonably high conductivity, as recorded in Table 1, which include polyaniline (PANI), polypyrrole (PPy), poly(3,4-ethylenedioxythiophene) (PEDOT), polythiophene (PT), polyacetylene (PA), polyparaphenylene (PPP) and others, as illustrated in Figure 2. CPs show semiconducting characteristics before a “doping” process, which involves oxidizing or reducing CPs. The electrical properties can be controlled by doping process that significantly rises the number of charge carriers in CPs. Since the discovery of conducting PAc, a variety of CPs [13]
At present it is possible to create conducting polymers with a diverse range of chemical, electrical and other properties. Due to this collection of dynamic properties conducting polymers have found uses in a range of different application in areas such as within mechanical actuators, corrosion protection, anti-static coatings, sensors, membranes, energy storage and conversion devices, polymer photovoltaics, light emitting diodes and display technologies [14-18].

Figure 2 Chemical structures of representative conducting polymers.
Table 1
Conductivity of common conducting polymers
|
Polyaniline (PANI) |
30–200 |
n, p |
|
Polypyrrole (PPy) |
10–7500 |
p |
|
Poly(3,4-ethylenedioxythiophe) (PEDOT) |
0.4–400 |
n, p |
|
Polythiophene (PT) |
10–1000 |
p |
|
Polyacetylene (PA) |
200–1000 |
n, p |
|
Polyparaphenylene (PPP) |
500 |
n, p |
|
Polyparaphenylene sulfide (PPS) |
3–300 |
p |
|
Polyparavinylene (PPv) |
1–1000 |
p |
|
Polyisothionaphthene ( PITN ) |
1–50 |
p |
Reproduced from references [19–20].
The electrical conductivity of polyacetylene can be improved by largely upon doping. But it is highly susceptible to atmospheric oxidation which limits its applications. The electrical conductivity of polyaniline as well as polypyrrole can be tuned from the insulating to the metallic region upon suitable doping. These two conducting polymers, especially polypyrrole exhibits high electromagnetic shielding (EMI shielding) properties. Polythiophene and poly (phenylene-vinylene) are more known for their photoluminescent as well as electroluminescent properties [21].
The development of conducting polymers can be divided into three periods; the first generation of conducting polymers are made conductive by incorporating conductive filler and/or additives.
The second generation of conducting polymers conduct electric current themselves without the need for any additives. The next generation of conducting polymers which is the third generation are mostly insulators or poor conductors but can be made conductive by incorporating conductive filler and/or additives. The conductivity of such doped polymers depends on the nature of the dopants and the level of doping.
It is convenient to divide conducting polymers into three main categories based on their chemical properties.
Amphoteric polymers i.e. the polymers whose neutral chains can be converted either into polycarbonium cations or polycarbonions via redox reaction. As a result the polymers may exhibit either p-type or n-type conductivity.
Polymers whose neutral chains can be oxidised to polycarbonium cations but cannot be partially reduced to polycarbonions.
Polymers possessing strong basic centres in their conjugated backbones. These can be converted into salt-like, conductive compounds via an acid based reaction with a protonic acid.
Synthetic Strategies of Conducting polymer composites
Conducting Polymers are strategically synthesized through oxidative coupling of monomers. In the case of polymerization, the first stage is the oxidation of the monomer, which yield a radical cation, which then reacts with another radical cation, to form dimer [22]. Hence, an obvious classification is the initiation process of polymerization, which includes three major steps: oxidation, coupling and propagation. These three steps serve as integrated and key components which determine the physical and chemical properties of CPs. The oxidation stage can be activated through several methods which includes chemical, electrochemical, and photo-induced oxidation, with each having its own merits and demerits as shown in Figure 3. In the first method, chemical oxidants (such as ferric chloride or ammonium persulfate) are applied to oxidize the monomer. In the second method, the monomers are oxidized electrochemically, and in the third method, light is required to oxidize the monomer with a photo initiator [22,23].

Figure 3. Schematic comparing the chemical, electrochemical, and photopolymerization mechanisms. Each method has been evaluated in term of variables (V), in which a low value means there are many key variables in the synthesis process; cost effectiveness (C), in which a low value corresponds to high cost; morphology control (M); time of reaction (T); scalability (S); and the resulting purity (P) of the materials [23].
Chemical oxidative polymerization is a common method employed to synthesise conducting polymers in bulk form, such as polyaniline, polypyrrole etc. It involve either condensation polymerization (step-growth mechanism) or addition polymerization (chain-growth mechanism). Here the monomer is dissolved in the dopant acid solution .The oxidant dissolved in appropriate solvent (water is the commonly used solvent) is added drop wise to the mixture with continuous stirring for 4-5 hours, after which The precipitate obtained is filtered, washed and dried. For the synthesis of conducting polymers such as polyacetylene, more complicated methods such as catalytic polymerization, non-catalytic polymerisation, isomerization, Durham method, dehydrochlorination and dehydration are used [24]. The main benefit of chemical synthesis is that it not only give room for several possible routes to synthesize different CPs, but also allow the production of these materials in large quantities, which is presently not obtainable with electrochemical synthesis. Moreover, chemical synthesis methods have more options in the area of covalent modification of the CP backbone [23,24].
The electrochemical polymerisation can be carried out by employing either a constant current, a constant potential or potentiodynamic techniques, it is a relatively straightforward synthetic method for fabricating CPs. Conducting polymers can be electrochemically polymerized due to their conductivity properties. In general, electrochemical polymerization is employed using a three-electrode

Figure 4. Mechanism for chemical polyerization of PPy [26].
system (working, counter, and reference electrodes) in a solution comprising a monomer, an electrolyte, and appropriate additives. A number of crucial factors must be considered, including the electrolyte, deposition time/method (continuous versus pulsed) and applied potential. All these parameters has an influence on film morphology, conductivity, as well as the mechanical properties, which directly influence the usefulness of the material for many applications. Electrochemical synthesis is flexible in terms of the dopant that can be incorporated from the electrolyte and is a useful tool for the preparation and characterisation of small films or coated electrodes [25]. Compared to chemical oxidation, electrochemical oxidation offers a number of advantages: ability to incorporate various dopants, easy control of the microstructures, sequential deposition to produce layered structures, and the ability to form copolymers. The monomer is dissolved in a solvent containing dopant and oxidised at the surface of the electrode by applying an anodic potential. The solvent and electrolyte have to be stable at the oxidation potential. Organic solvents, such as acetonitrile, which have large potential windows are usually employed [26].

Figure5. Mechanism for electropolymerization of PPy [26].
The significant differences between chemical and electrochemical methods have been investigated by many researchers. In the case of electrochemical methods, very thin CP films of close to 20nm can be produced while powders or very thick films can be produced using the chemical technique [27]. However, this idea is being challenged. A lot effort has been spent on experimental research to overcome this difference, and at the moment, the chemical method can produce thin CP films through modification of the nature and concentration of the oxidizing agent. Though chemical oxidative polymerization can also be used, but the electrochemical method remains the most preferred method for CP thin films because employing an appropriate electrical potential allows the production of high-quality films with the desired thickness [28]. A number of problems associated with the electrochemical method remains the comparatively poor reproducibility of bulk CPs and the fact that it is somewhat difficult to remove the grown film from the electrode surface. Several types of CPs can be produced via chemical polymerization methods, however electrochemical production is only suitable for those designs by which the monomer can be oxidized through a potential to produce reactive radical ion intermediates for polymerization; several standard CPs like PPy, PT, PANI, PEDOT can be designed both chemically and electrochemically. Till recent, due to the chemical diversity of the studied monomers, a general scheme for the electrochemical approach cannot be made available. A good number of reports have been made on the comparison of chemical and electrochemical polymerization methods for the same monomers [29,30], with results indicating that the electrical conductivities of CPs produced electrochemically were higher than those of CPs produced through chemical polymerization. Another work by Gorey et al. revealed that in electrochemical methods, the polymerization time is quicker than that of chemical methods approximately a few minutes as against a few hours), while chemical growth can offer more homogeneous morphologies than the electrochemical route [31].
Another method used in the polymerisation of the monomers is the Photopolymerization in which the monomers are exposed to ultraviolet (UV) light, visible light, laser-generating radicals (photochemical reaction), or holes (photoelectrochemical reaction). Examples of photopolymerization can be categorize into two namely: direct photopolymerization and photosensitizer-mediated polymerization. Direct photopolymerization involves the absorption of energy of illumination and also decomposition of the monomers into radicals, which is similar to free radical polymerization. However, CPs cannot be produced by direct photopolymerization due to the fact that they have a more positive oxidation peak potential than the redox potential of the photosensitizers [32].
Another commonly employed method to synthesise soluble conducting polymers is the copolymerization technique. This method provides additional advantage such that it is possible to blend the distinct advantages of various polymers into one system [24]. For instance, this procedure is mostly used to impart debility to nonreactive fiber producing polymers such as acrylics [21,24]. In this method, the polymer is copolymerized with monomeric groups that form anionic moieties at the proper pH. The product produced can be readily dyed with cationic dyes.
Usually there are four basic different methods of copolymerization [21]. They include,
(a) Insitu polymerization of both monomers to form a random copolymer
(b) Grafting of the solubilizing polymer onto the backbone of the insoluble polymer
(c) Grafting of the insoluble polymer onto the backbone of the soluble polymer
(d) Combination of both soluble and insoluble polymers to form a block copolymer by various synthetic methods
(e) Insitu polymerization of an insoluble polymer onto the matrix
of a soluble polymer. This include composites of polyaniline and polypyrrole produced by in situ polymerization in matrices such as poly (vinyl chloride) (PVC), polyvinyla1cohol (PVA), poly
(vinylidinechloride-co-trifluoroethylene) and brominated poly (vinyl carbazole) [33,34].
(f) Counterion-induced processibility of conducting polyaniline(PANI)
3. Conducting polymer-based composites for supercapacitor applications
Due to easy production and low cost of conducting polymers, they have been widely researched as supercapacitor electrode material, they possess a relatively high conductivity and capacitance and also equivalent series resistance when compared with some carbon based electrode materials. For CPs, There are different electrode configurations that can be used, the includes the n/p type configuration, having one negatively charged (n-doped) and one positively charged (p-doped) electrodes, offers a high energy and densities, although due to lack of n-doped conducting polymer electrode materials have limited psuedocapacitors from reaching their potential [36].
In conducting polymers reduction-oxidation process is used to store and release charge. If oxidation occurs, ions are been transferred to the polymer back bone. If reduction occurs also known as de-doping, in that case ions are released back into the solution [18]. Due to the reduction-oxidation in conducting polymers these causes a mechanical stress which in turn limits the stability through many charge-discharge cycles [36].
Considering the different types of conducting polymer, PANI is considered as the most promising supercapacitor electrode material because of its high conductivity, easy synthesis, excellent capacity for energy storage and low cost [37]. Nevertheless, because of the repetitive cycles (examples, charge/discharge process) swelling and shrinkage, PANI is subject to rapid degradation in performance. To avoid this limitation, combining PANI with carbon materials has proved to reinforce the stability of PANI as well as maximize the capacitance value [38, 39].
CPs can be p-doped with anions or n-doped with cations. The mechanism of electrochemical doping of a conducting polymer is described in Figure 6. Electrochemical p-doping of conducting polymer occurs by removing electrons from the polymer chain through the external circuit and entering of anions from the electrolyte into the polymer to balance the positive charge. Reverse of this mechanism accounts for the electrochemical n-doping of conducting polymers. Electrons enter the polymer chain through the external circuit and the cations from the electrolyte enter the polymer to balance the negative charge. PPy and PANi can only be p-doped in a supercapacitor system because of the very negative potentials needed for n-doping, which is above the reduction potential limit of the electrolyte [40]. PT and its derivatives can be both p- and n-doped [41, 42].
Three device configurations have been developed for supercapacitors solely composed of conducting polymers; [26].
(1) Type I (symmetric) using the same p-dopable polymer in both electrodes. When the device is fully charged, one electrode is fully doped and the other one is undoped. The voltage of this type of device is limited to 0.8-1 V.
(2) Type II (asymmetric) which uses two different p-dopable polymers with different redox potential windows, which includes polypyrrole and polythiophene. Compared to Type I, this type of device delivers higher voltage of ~1.5 V.

Figure 6. Schematic representation of electrochemical doping of conducting polymers [43]: (a) p-doping and (b) n-doping.
(3) Type III (symmetric) using the same polymer electrodes that can be p-doped and n-doped. As the device is fully charged, one electrode becomes fully p-doped and the other one becomes fully n-doped, resulting to a high cell voltage of more than 3V. Much higher specific capacitances have been obtained for CPs compared to carbon materials. Fan et al. prepared a highly porous PPy electrode on Ti foil via the cyclic voltammetry method. The resultant PPy showed a specific capacitance of about 450 F g-1 [44]. Perchlorate doped PPy showed a specific capacitance of 344 or 355 F g-1, respectively [45]. PANi was reported to show a specific capacitance higher than 500 F g-1 [46]. A derivative of PTh, poly(tris(4(thiophen-2-yl)phenyl)amine), can even achieve a specific capacitance greater than 990 F g-1 [47]. However, the major issues with CPs based electrodes is that og the structural degradation due to swelling and shrinking of CPs during long term cycling, resulting to electrochemical performance fading. It is suggested that nanostructured CPs [48] or compositing with carbon based materials [49-51] can enhance the cycling stability.
Significant challenges in supercapacitors
In recent times, CPs are considered to be one of the most promising electrode materials for supercapacitor application because of their high flexibility and the fact that they are easy to fabrication [52]. Morphology of CPs plays a significant role in their electrochemical performances. In literature, several CPs of various forms, including bulk powder, nanorods, nanosheets, and nanowalls, together with their versatile availability, have been produced and applied successfully. But however, for supercapacitor applications, Insolubility and intractability remains the major challenges obstructing the use of CPs in energy storage, other problems includes diffusion issues, cycling, and stability. These challenges of CPs had traditionally been tackled through dispersing the polymer in solutions using a series of steps geared towards removing the aggregates and ensure a stable suspension [53].
Conducting polymer-based binary composites for supercapacitor applications
Conducting polymer like PANI, PPy and PTH are low cost and good materials for supercapacitor applications. However, due to volumetric shrinkage suffered by some of these CPs during dedoping processes, which results to reduction in cycling stability, recent research has focus on improving the performance of CPs on these areas by making composites with particulate reinforcement with metal oxides and ferrites, fibre reinforcement with some carbon based materials. CPs have been composited with any of these materials forming either a binary nanocomposites or a ternary nanocomposites. In this way, the capacitive performances of such advanced functional and hybrid materials have been improved by synergistic effects, particularly for supercapacitor applications [54].
3.3.1. Conducting polymer-Carbon based binary composites
Supercapacitors with high power density and excellent cycling stability are the essential substitutes in energy storage devices with the potential to meet increasing energy demands and environmental concerns. Various carbon materials including activated carbon (porous carbon), ordered mesoporous carbons, carbon aerogels, carbide-derived carbon, graphene, and carbon nanotubes remain the most common and important electrode materials for supercapacitors which are also referred to as Electrical double layer capacitors (EDLC). The first well documented report defining the concept of an electrochemical capacitor was done in 1957 by Becker, when he used carbon with a high specific surface area (SSA) coated on a metallic current collector in a sulfuric acid solution. Another case was in 1971, when NEC (Japan) developed aqueous-electrolyte capacitors under the energy company SOHIO which was used as power saving units in electronics, and this effort can be regarded as the starting point for electrochemical double layer capacitors (EDLC) in commercial devices [55].
Electrode materials for supercapacitors are carbon materials, which has many advantages, such as large surface area and high conductivity. Others includes easy availability and environmental friendliness. To achieve superior capacitive properties, carbon materials are composited with conducting polymers with tailored nanostructures. As some major electrode materials for supercapacitors, carbon materials provide efficient supporting substrates as well as highly conductive pathways. Typically, nanostructured carbon materials possessing large porosity and high surface area can provide effective decoration sites to support the formation of nanostructured conducting polymers. Nanostructured carbon materials are also better alternatives for increasing performances when composited with conducting polymers, hence, the introduction of nanostructured carbon materials with one-dimensional (1D), two-dimensional (2D) and three dimensional (3D) architectures into conducting polymers has a significant, positive impact on the electrochemical capacitive performance [56].
3.3.1.2. Conducting polymer- graphene based binary composites
Composites of graphene and its derivatives with CPs can lead to drastic enhancement of the electrochemical properties of CPs and hence have great influence on the morphologies, electrical properties, and the structural stabilities of the CPs. Nevertheless, for a graphene/CPs supercapacitor, there are many factors which defines the final supercapacitor performance which includes its specific capacitance, cycling stability, charge and discharge properties, etc. others includes electrolyte, dispersion/aggregation of the graphene in the composites, different properties of graphene used, intrinsic properties of individual CPs, and polymerization method, etc. there are different types of benefits and disadvantages in using the different types of CPs to form composite, PEDOT has the largest molecular molar mass, hence, the specific capacitance of the graphene/PEDOT composites supercapacitor is normally smaller than the ones formed with either PANI or PPy. On the other hand, the cycling performance of PPy is normally the worst among the three CPs due to the fact that the larger particles formed in the PPy film make it less porous than PANI or PEDOT, the conductivity comparison of these CPs is PEDOT>PPy>PANI which can affect the supercapacitors [57]. Nevertheless, CPs and graphene derivatives are fabricated and employed as the hybrid kind of supercapacitor. Graphene which can be seen in the form of reduced Graphene Oxide (rGO) has been found to be extremely attractive for application in supercapacitors due to its unprecedented properties which includes excellent electrical and electrochemical performances. Some scientists have designed rGO-wrapped PANI nanofiber composites via assembly of negatively charged graphene oxide with positively charged PANI nanofibers in aqueous dispersions followed by the reduction of GO to rGO. The capacitance of the composites were found to be 250 F g1 at 0.5 A g1 in a 1 M Et4N+BF4/propylene carbonate organic electrolyte and only 26.3% of the capacitance decreased over 1000 cycles [56].
One of the most widely used conducting polymers with graphene oxide (GO) sheets for supercapacitors is the PANI (Fig. 8). This GO, comprises of oxygenated groups which includes epoxides, hydroxyl groups, and carboxyl functional groups on its basal planes and edges, they also have better compatibility with polymers and has been employed in fabricating conducting polymer-based composites due to its amazing morphology and strong hydrophilicity [56]. In addition, in the course of oxidative polymerization, some monomers like aniline are oxidized to PANI, and simultaneously, which causes the removal of oxygen and GO is reduced to graphene, creating good conducting networks [58,59]. Furthermore polymer tailored graphene oxide sheets have been utilised with acid-oxidized multi-walled carbon nanotube (MWCNT) to produce hybrid carbon film at an average specific capacitance of 120 Fg-1 and high scan rate of 1 Vs-1 [3].

Figure 7. Schematic representation of a supercapacitor.
3.3.1.3. Conducting polymer-CNT based binary composites
CNTs, exhibiting high mechanical strength, good chemical stability and excellent electrical conductivity, have been highly researched and utilized for supercapacitors [60,61]. Also research have shown that randomly arranged CNTs within the conducting polymer matrix possess a synergistic effect on the final capacitive performance. Moreover, ordered structured CNTs can significantly improve the electrochemical performance. However, there are a few limitations for CNTs, such as poor processability and lack of chemical properties. Therefore, CP/CNT nanocomposites would provide a meaningful aspect to improve or extend properties of both nanomaterials. For example, the electron/hole transport will be improved due to the strong interactions. Many scientists have tried to explore effective methods to produce CP/CNT nanocomposites. However, electrochemical polymerization remain the most convenient method to produce CP/ CNT nanocomposites due to the fact that the morphology and properties of the nanocomposites can be well controlled by this method [13]. CNTs have shown encouraging flexibility and are promising as free-standing and flexible electrodes. These composite electrodes can be fabricated by some techniques like solution-blending method, in situ polymerization method. Some researchers have equally prepared robust free-
standing CNT/conducting polymer composite electrodes by a filtration method, which can be easily scaled up. These composite electrodes exhibits an improved capacitance. This method provides a simple, low-cost and high-throughput approach for large-scale production of CNT/conducting polymer composite electrodes. Other methods which has been used by some researchers includes mechanical methods which has been used by some researchers includes mechanical methods.

Figure 8. PANI/GO composite.

Figure 9. a) Schematic diagram for making the flexible PANI/CNT super capacitor (b) CNT/PANI [62].
3.3.1.4. Conducting polymer-porus, mesoporous based binary composites
Porous, mesoporous and other 3D carbon materials have attracted tremendous attention as supercapacitor electrode materials because of their excellent properties which includes, good conductivity, high surface area, and good electrolyte accessibility. In addition to these attractive characteristics, high surface area and interconnected architecture, unlike the 1D and 2D carbon materials, allow 3D carbon materials to possess improved conductive networks [63,64]. The 3D carbon materials, such as carbon fibers, carbon cloths, carbon aerogels and other porous carbons, have more active sites for the growth of the conducting polymers. Recently researched, carbon materials electrodes for supercapacitors have shown specific capacitances that are not directly proportional to their surface area, [65,66] and this is due to the fact that not all micropores in the electrodes are necessarily accessible to the electrolyte ions [67,68]. Therefore, 3D carbon materials with ordered pores would be able to reach a maximum capacitance due to the perfect match between the pore sizes and electrolyte ion sizes.
3.3.2. Conducting polymer-metal (metal oxides, metal hydroxides, sulfides, phosphides, etc) based binary composites
The improvement in the cyclic stability of conducting polymer electrodes has become a serious issue to be focused on. therefore, the fabrication of conductive polymer composites with a combination of metal oxides, metal hydroxides or metal sulfides have been found to improve the volume change during the repeated redox cycles and also enhanced the mechanical stability as well as the specific capacitance of the conducting polymers [56].
Supercapacitors made from metal oxides/hydroxides as electrode materials has been found to possess higher energy densities compared with those made from carbon materials and with enhanced cyclic stability than the conductive polymers [69,70].
Moreover, the electrochemical processes for metal oxides/hydroxides have equally be found to occur both on the surface and in the bulk of the electrode materials, which resulted to an increase in capacitances [71]. Series of conductive polymer–metal oxide/hydroxide composites that shows high specific capacitances and enhanced cyclic stabilities in supercapacitors has successfully been produced by some scientist [72-74]. The conductive polymers in these composites can prevent the agglomeration and restacking of metal oxide/hydroxide particles through the space steric hindrance and electrostatic effect, which allows the metal oxide/hydroxide particles to be uniformly dispersed into the conducting polymer matrix. The contact area between the electrode and electrolyte can be enlarged by the conductive polymer–metal oxide/hydroxide composites, which will result to enhancement on the adhesion between the current collector and electrode materials.
The commonly used metal oxide/hydroxide materials, includes ruthenium oxide (RuO2), manganese dioxide (MnO2), nickel oxide (NiO) and nickel hydroxide (Ni(OH)2). The lower cost of production and use of a milder electrolyte make them a feasible alternative.
3.3.2.1. Ruthenium oxide (RuO2)
RuO2 in both amorphous and crystalline forms is essentially important in both theoretical and practical purposes, because of its unique combination of characteristics, which includes catalytic activities, metallic conductivity, electrochemical reduction-oxidation properties, high chemical and thermal stability and field emitting behaviour. Thus having these properties RuO2 has been found to be useful as an electrode material in supercapacitors [75]. Among the many metal oxide that are used as electrode materials, RuO2 has had the most success as a result of its advantages of long cycle life, wide potential window of high specific capacitance, highly reversible reduction-oxidation reaction, and metallic type conductivity. For supercapacitor application Gujar and co-workers produced RuO2 electrochemically using electrodeposition method. The resulting electrodes were found to be stable for large number of cycles yielding a specific capacitance of 498 F/g at a scan rate of 5mV/s [76]. Lee and co-workers reported RuO2/PEDOT nanotubes with a high specific capacitance of 1217 F g-1 and a high power density of 20 kW kg-1 from the high specific surface area and fast charge/discharge of the tubular structures [77]. However, despite the high specific capacitance of RuO2, some factors has restricted its mass production and widespread application as an electrode material for EDLC, such as its high cost and toxic nature. Hence, the combination of metal oxides and hydroxides with inexpensive conducting polymers can considerably reduce the cost and also maintain the excellent performance [78–82].
3.3.2.2. Manganese dioxide (MnO2)
Manganese dioxide (MnO2) has been found to be useful as an electrode material for supercapacitors because of its low cost, excellent capacitive performance in aqueous electrolytes, with specific capacitance (1100 F g-1 of theoretical capacitance), and More importantly, environmentally friendly nature and readily availability of MnO2 will significantly lower its cost when compared with other noble metal oxide. [83-86]. However, the problem of MnO2 is its relatively low electric conductivity, which will affect its performance as capacitor [87]. But more and more attempts have been brought out to solve this problem. A typical approach is to composite MnO2 with conducting Materials, Hence, forming composites of MnO2 with conducting polymers as electrode materials for supercapacitors has attracted intense attention. Dr. Xiaodong and co-workers reported a remarkable research on the enhancement of MnO2 capacitance by composited it with polypyrrole. The specific capacitance of MnO2 powder can reach 294 F/g if the size of MnO2 decreases to a nanoscale. They described a method to produced ultrafine MnO2/polypyrrole nanorod composite powders at room temperature. In this synthesis method, pyrrole monomer is a reductant to reduce Mn7+ ions in KMnO4 solution to Mn4+ ions in the form of MnO2 crystal. At the same time, the pyrrole monomers go through the process of oxidative polymerization to produce conductive polypyrrole [88]. Dr. Jaidev and co-workers report a facile approach for synthesis of a novel Polyaniline (PANI)/MnO2 nanotube (MNT) hybrid nanocomposite by in situ polymerization. This nanocomposite combines the advantages of MnO2, low cost and high power density, with the PANI’s high electrical conductivity [89]. Another study on MnO2-based electrode with the addition of PPy showed the improved speci¯c capacitance of the MnO2-PPy nanocomposite (290F/g) compared to that of pure MnO2 electrode (221F/g). The enhancement is attributed to a combination of the improved conductivity effect and the high specific capacitance of PPy [90].
3.2.2.3. Conducting polymer/nickel oxide–hydroxide composites.
Another promising electrode material for supercapacitor is the nickel oxide (NiO) and nickel hydroxide (Ni(OH)2) due to its environmental friendliness, easy synthesis as well as low cost. Using electrochemical strategy nickel hydroxide was transformed into nickel oxide due to some of its unique properties which include reliability, simplicity, accuracy, low cost and versatility. The resulting method produced an ultra-high specific capacitance of 1478 F/g in 1M KOH aqueous solution electrolyte [91].
Zhao et al. used the in situ method to synthesize flower-like PANI–NiO nanostructures on nickel foams as binder-free electrodes, which can attain a specific capacitance of 2565Fg-1 at 1Ag-1 and maintain 70% of the capacitance with the current density increasing 10 times [92]. Hu et al. in their work prepared porous NiO/Ni(OH)2 composite (PNC) encapsulated in 3D interconnected PEDOT nanoflowers on metal wires (Cu–Ni alloy) via a mild electrochemical route. The surface of PNC is covered with a protecting thin layer of steady PEDOT, to enhance the electronic conductivity as well as the structural stability of the NiO/Ni(OH)2/PEDOT composites. The 3D flower-like nanostructure reached a high specific capacitance of 404.1 mF cm-2 with a current density of 4 mA cm-2 and also a long-term cycling stability with 82.2% capacitance retention after 1000 cycles [56].
3.2.2.4. Conducting polymer/other metal oxide–hydroxide composites.
Many other metals oxides such as tin oxide (SnO2), vanadium oxides (V2O5), cobalt monoxide (CoO), etc have also been investigated and incorporated with CPs as electrode materials for EDLC.
Hu some researchers showed that the complementary properties of SnO2 nanoparticles embedded within PANI exhibited excellent capacitance of 305F/g with a specific energy density of 42Wh/kg. The improvements of the capacitive performance is believed to be attributed to the presence of SnO2 nanoparticles embedded within PANI chains, increasing the electrode–electrolyte interfacial area for insertion as well as extraction of ions [93]. V2O5 has equally attracted intense research attention for its variable oxidation states, surface/bulk redox reactions and layered structures, which facilitate efficient ion dispersion [94,95] Liu and his group produced large surface area V2O5–PANI composite nanowires by electrodeposition method, this method can promote the effective contact of electrochemical active materials with the electrolyte and achieve a high specific capacitance of 412Fg-1 at 4.5mA cm-2 [96]. CoO, another electrochemically active material, has also been explored for pseudocapacitive energy storage by elaborate incorporation with conducting polymers to tackle the problems associated with capacitance and stability. Liu and his group produced a well-aligned CoO/PPy nanowire array grown on nickel foam as a pseudocapacitive electrode. The assembled asymmetric supercapacitor showed a high specific capacitance of 2223 F g-1 and excellent cycling stability due to the shortened ion diffusion channels and the highly conductive nanowire arrays [97].
3.2.2.5. Conducting polymers with metal sulphides
Most recently, major research efforts are focused on exploring metal sulphides which are greatly abundant in nature and can equally undergo redox transitions with different valence states of metal ions to improve energy density of electrochemical capacitors. Particularly, increasing both capacitance and operating voltage of electrochemical capacitors are of significant importance since energy density is proportional to capacitance and squared voltage [98]. Some nanostructured metal sulfides, such as MoS2, NiCo2S4, CuSx, NiSx, CoSx, have received a great deal of attention due to their excellent redox reversibility and relatively high specific capacitances and have been integrated with conducting polymers as a new type of energy storage materials [99-102]. MoS2 with its double layer charge storage and also the range of oxidation states of Mo, from +2 to +6, is expected to possess excellent electrochemical capacitive properties.
An improved specific capacitance of 552 F g-1 at 0.5 A g-1, together with an outstanding rate capability of 82% from 0.5 to 30 A g-1 was achieved by Liu et al. by using an in situ oxidative polymerization techniques to prepare a 3D tubular MoS2/PANI nanostructure with PANI nanowire arrays vertically aligned on the external and internal surfaces of 3D tubular MoS2 [103].
Lei et al. in their work successfully produced CuS microspheres where PPy was uniformly injected into the intertwined sheet-like subunit and coated onto the CuS surface, and it exhibited a specific capacitance of 227 F g-1 and excellent cycling stability [104]. However, a very high specific capacitance of 713 Fg-1 with an energy density of 239.0 W h kg-1 and a power density of 39.5 W kg-1 at a current density of 0.8 mA cm-2 was accomplished by Xu and his group when they produced a highly conductive PPy/NiS/bacterial cellulose nanofibrous membrane as a flexible electrode [105].
Conducting polymer-based ternary composites for supercapacitor applications
Recently, ternary nanocomposites which combined three components from CPs, inorganic materials (metal, metal oxides, etc.) and carbon nanomaterials have attracted the attention of many researchers due to their excellent performance over binary systems. In ternary structural design, several synergistic effects were identified in their application as the electrode material for EDLC [13]. Recently, a novel MnO2/PANI/carbon ternary composite in 1M H2SO4 was successfully synthesized by Yan and co-workers. With the 12% MnO2 loading, the maximum specific capacitance of 695F/g and the 88% capacitance retention were achieved around 1000 cycles. Their study demonstrated that the protective PANI nanolayer enables MnO2/carbon composites to be operated in acidic electrolyte [106]. Hou and co-workers reported that the specific capacitance of the ternary composite MnO2/CNT/ PEDOT-PSS reaches 200F/g with only less than 1% decay in specific capacitance after 1000 cycles. They suggested that the mechanical stability of the composite improved as a result of presence of the conductive CNTs which provide high surface for the deposition of very porous MnO2 nanospheres [107]. Furthermore, a high specific capacitance of 1020F/g has been achieved by some group of researchers from novel PANI/TiO2/graphene oxide. Here TiO2 nanoparticles serve as avenue for electrode to penetrate to three-dimensional graphene oxide-PANI networks, facilitating the Faradaic reactions of PANI [108].
4.1.1 Conducting polymer- Li-ion based composites for battery applications
Li-ion batteries are among the most promising, efficient and common high-energy-density systems used in electrochemical energy storage. The Li-ion battery technology formulate a dependable system for electricity storage exhibiting mainly high energy density and design flexibility. Widely used in “nomadic” electronic devices, such batteries also appear to be an important element to mitigate CO2 releases i) as a promising power source for advanced electric vehicles and ii) as a potential buffer energy storage system to manage the intermittent renewable energy resources (both on- and off-the grid). Thus the production and world use of Li-ion batteries is expected to keep increasing [109]. In recent times, the use of Li-ion batteries in common electronic devices, and also the interest for more effective and safer batteries, has increased tremendously. Batteries with great features like superior mechanical properties, higher efficiency, and smaller size are required for handheld electronics to continue with the fast increasing computing power, larger screens and thinner and lighter designs of such devices. Also, there is increasing interest for polymer-based batteries to be integrated with flexible, soft and microelectronics. There has also been a significant increase with concerns regarding the issues associated with such batteries. The utilisation of flammable organic solvents as electrolyte, development of lithium dendrites, and large volume change as a result of poor structural stability are among the problems associated with Li-ion batteries [110].
The main theory of Li-ion batteries is shown in Figure 10. In the figure, an arrangement of a negative lithium intercalation material (anode) by another lithium intercalation material (cathode) having a more positive redox potential produced a Li-ion transfer cell. Anode and cathode are disconnected by the electrolyte which is an electronic insulator but also a Li-ion conductor. On charging, Li-ions are released by the cathode and placed at the anode. When the cell is discharged, LI-ions are extracted by the cathode and inserted into the anode [111].
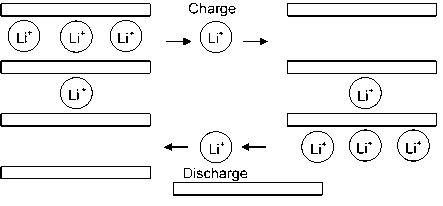
Figure 10. Schematic illustration of a lithium-ion battery.
CPs are promising materials for organic-inorganic hybrid composites for Li-ion batteries due to their excellent characteristics which includes high coulombic efficiency and electrical conductivity, which helps them to be cycled as many times as possible with little or no degradation. Conductive polymers exhibits several advantages, like good processibility, low cost, convenient molecular modification, and light weight when applied as electrodes. However, poor stability during cycling and low conductivity in reduced state inhibit their further applications in lithium-ion batteries. Nevertheless, the adoption of nanostructured conductive polymers can partly overcome this problem, owing to their high surface and faster diffusion kinetics of Li ions. In addition, CPs composites with inorganic compounds have received an increasing interest as promising matrices for the confinement of LI-ion batteries. This is because CPs can synergistically with inorganic compounds, significant improve the electrode life time, rates, and voltage, mechanical and thermal stability has been received [112]. CPs can be used as both anodic and cathodic materials, but are mostly used as cathodes in Li-ion batteries. Different conductive polymers exhibits quite different energy densities and power densities because of their chemical structures and physical properties [113]. For instance, PPy-based electrodes has energy densities of approximately 10–50 W h kg-1 and power densities of 5–25 kW kg-1; PANI-based electrodes show energy densities of 50–200 W h kg-1 and power densities of 5–50 kW kg-1; PTh-based electrodes show energy densities of 20–100 W h kg-1 and power densities of 5–50 kW kg-1 [113, 114]. Ordered PANI nanotubes doped with HClO4 synthesized by Cheng and co-workers was utilized as positive electrode materials exhibited better performance than the commercial PANI powders in lithium-ion batteries [115]. The Li–PANI battery attained a high practical discharge capacity of 75.7 mA h g-1 and maintained a 95.5% of the highest discharge capacity after 80 cycles. However, the capacity and power density is still relatively low and the stability of organic materials remains a serious problem.
4.1.2. Conducting polymer- Na-ion based composites for battery applications
To tackle the increasing demands for green and sustainable energy, Na-ion batteries are being considered as better alternative to current Li-ion technology, due to their material availability, low-cost and environmental friendliness. Recent researches on Na-ion battery are mostly geared towards the production of inorganic Na intercalation materials. Redox-active polymers has been widely researched and found to be a good choice of electrode-active materials for Na-ion batteries due to their structural diversity and materials sustainability. As a flexible framework, organic polymers can take larger Na ions reversibly without much spatial problems, hence helping to achieve a fast kinetics for Na insertion and extraction reactions. In principle, a Na-ion battery can be designed through the use of a pair of organic cathode and anode which always have sufficient potential difference and can be coupled well to carry out a battery reaction. Such an all-organic Na-ion battery would be greatly attractive for large scale electric storage applications because of its low cost and eco-friendliness [116]. Very recently, Wenwen et al. reported an all-organic Na-ion battery using p-dopable polytriphenylamine as cathode and n-type redox-active poly(anthraquinonyl sulphide) as anode, without suing the transition-metals as was used in conventional electrochemical batteries. This Na-ion battery showed an output voltage of 1.8 V and attained a significant specific energy of 92 W h kg-1. As a result of the structural flexibility and stability of the redox-active polymers, this battery was observed to have an excellent rate capability of 60% capacity release data at a very high rate of 16 C(3200 mAg-1) and also show an excellent cycling stability with 85% capacity retention after 500 cycles at 8 C rate.
4.1.3. Conducting polymer- Mg-ion based composites for battery applications
Lithium ion batteries has been the subject of much global attention in the continuing development of advanced energy storage technologies. Till recent, these batteries offer the best combination of energy capacity per gram, cost, and long term cycle stability. Nevertheless, Li-ion batteries have several limitations which includes mostly high cost of materials used for the production of the batteries, while capacity is mostly limited by the monovalent nature of the Li-ion. From the theoretical point of view, all the problems associated with Li-ion battery technology are overcome by magnesium batteries. Magnesium being a divalent ion, allowing for double the theoretical capacity of a Li-ion cell. In addition, magnesium is much more readily available than lithium, thereby reducing the production cost [117]. Mg metal is also much less reactive in air than both Li and Na which makes it more convenient to handle; more so it has a significantly higher volumetric capacity (that is, 3832 mAh cm-3, as compared with Li and Na which has 2062 mAh cm-3 and 1136 mAh cm-3 respectively); and smooth, dendrite-free Mg deposition and close to a 100% columbic efficiency (CE) for plating/stripping have been shown in certain electrolytes. These discoveries are possibly transformative since dendrite formation and low columbic efficiency have been long age problem with Li metal battery development [118]. Over the past few years, remarkable progress has been recorded in the area of rechargeable Mg battery field, especially with electrolyte design and fabrication. Magnesium forms a “truly” passivating film in contact with oxygen or conventional electrolytes (i.e., mixtures of simple Mg salts and aprotic solvents, similar to those in Li-ion batteries [119]), which hinders Mg2+ transfer [120–122]. Hence, a rechargeable Mg battery involves electrolytes in which no “solid electrolyte interphase” (SEI) or a weakened passivation layer is made on the Mg metal surface to enable highly reversible Mg plating/ stripping [118]. Yuyan et al. reported a nanocomposite polymer electrolyte based on poly(ethylene oxide) ( PEO), Mg(BH4)2 and MgO nanoparticles for rechargeable Mg batteries. Cells with this electrolyte were observed to have remarkable columbic efficiency of 98% for Mg plating/stripping and a high cycling stability. Via joint experiment-modeling investigations, a connection between improved solvation of the salt and solvent chain length, chelation and oxygen denticity was established. Following the same development, the nanocomposite polymer electrolyte is used to enhance the dissociation of the salt Mg(BH4)2 and thus increase the electrochemical performance [118].
5. Conclusions, future prospects and challenges
A comprehensive overview of conducting polymer composites for advanced electrode materials for EDLC and batteries has been examined. The main problems associated with conducting polymer electrodes includes poor rate and cyclic stability. These problems can be reduced by effective combination of conducting polymers with other materials to form composite electrodes materials. Combination of these composite nanostructures in forming the conducting polymer composites ensures the composite electrodes have a largely improved energy density and excellent cycling stability. Nevertheless, at the present stage, the capacitances of the conducting polymer composites achieved are insignificant compared to their theoretical values. Although the boosted performance after the composition of conducting polymers with metal oxides/hydroxides/ sulfides, there is still plenty of room to further improve the capacitance features. Also, while some reports have been studied, the design and production of ternary conducting polymer composite electrodes for use in supercapacitors and batteries has not being thoroughly explored. Such hybrid nanostructures in a multiphase system could be more reliable for better-working supercapacitors and batteries. In order to take full advantage of both energy storage mechanisms of electrochemical double layer capacitance and pseudocapacitance, other carbon materials, for example cheap carbon materials, can be used to ultimately improve the specific capacitance, energy density and power density. Also, the nature of the CPs provides them with excellent structural diversity, high flexibility and great durability. Additionally, the electrochromic characteristics of conducting polymers may probable be a superior advantage for their potential use in smart supercapacitors. With vigorous and high research interest and developments in conducting polymer composites for the last decade, conducting polymer composite electrodes are expected to play a leading role in flexible, smart and economical energy storage utilization in the future.


