Document Type : Review Article
Authors
Department of Pharmaceutical Chemistry, St. Joseph’s College of Pharmacy, Cherthala, Alappuzha, Kerala -688524, India
Abstract
One of the new global health risks is antimicrobial medication resistance. The creation of new and potent antimicrobial drugs is urgently needed due to the high mortality rate of this menace. One of the five-membered aromatic heterocycles subjected to the most inspection is the thiazole class. When it comes to therapeutic agents' antimicrobial action, the thiazole moiety is typically used as a fundamental framework. Many natural and synthetic thiazole derivatives have been found to exhibit significant biological activities. The current review gives a general overview of various thiazole synthesis techniques and describes various compounds with thiazole moiety with antimicrobial activity, which encourages further investigation into the development of thiazole-containing antimicrobial drugs.
Graphical Abstract
Keywords
Main Subjects
- Introduction
Antimicrobial resistance (AMR) has become one of the biggest hazards to public health, and World Health Organization (WHO) listed it among the top 10 global public health dangers to humanity. Global multisectoral action on AMR is urgently required to achieve sustainable development. Some of the key causes of this global hazard are inadequate controls on microbial infections, misuse, and overuse of antimicrobial drugs. In the 88 pathogen-drug combinations examined, it is estimated that 7 lakh deaths were directly related to the resistance (i.e. based on the counterfactual situation that drug-resistant illnesses were actually drug-susceptible) [1, 2]. If AMR is not adequately addressed, its effects could become more fatal than they are now. The situation now justifies the urgent need for new and powerful antibiotics.
Chemistry places a high value on the thiazole (1, 3-thiazole), a distinctive 5-membered heterocyclic ring with sulphur and nitrogen atoms. The thiazole ring can be found in numerous natural compounds, including peptide alkaloids, metabolites, and cyclopeptides, even though thiazole itself is not present in nature. Ritonavir, an anti-HIV medication, Tiazofurin and Dasatinib, as well as Ravuconazole, an antifungal medication, are a few examples of medications that include thiazoles [3-6].
Mechanism of Action of sulfathiazole: It prevents bacteria from synthesising the vitamin B complex that they need to flourish.
Cefpodoxime's bactericidal effect is brought on by its suppression of plasma membrane production. The active metabolite of cefpodoxime suppresses the peptidoglycan synthesis, the first component of bacterial cell walls, by preferentially binding to penicillin-binding protein 3.
Due to the development of medication resistance in resistant microbes, the discovery of antimicrobial agents is a subject of constant interest. Infectious disorders produced by bacteria, fungi, parasites, and viruses continue to pose a serious threat to public health despite extremely significant advancements in medicinal chemistry. It is obvious that therapeutic complication affects hospitalised patients, AIDS patients who are immunosuppressed, patients receiving anticancer treatment, and patients getting organ transplants the most. For medicinal purposes, there are numerous antibiotics and chemotherapeutics available. Despite this, modern classes of antibacterial drugs have become critically important in recent decades due to the rise of both old and novel forms of antibiotic resistance. Designing novel agents with a different mode of action is the only way to solve the resistance issue. Consequently, there will not be any cross-resistance with current treatments [7].
Therefore, the purpose of the current review is to summarize the various thiazole derivative productions and assess their antibacterial properties. The most recent publications are reviewed as a resource for future studies on the creation of novel chemical compounds with biological activity.
- Synthesis of Thiazole Derivatives
Hantzsch Synthesis: The earliest and best-known technique for synthesizing a thiazole ring involves cyclizing alpha-halocarbonyl compounds with several reactants that contain the N-C-S fragment (Scheme 1) [8, 9].

Scheme 1.
Gabriel Synthesis: At 170 C, acylaminocarbonyl compounds (4) undergo a cyclization reaction with phosphorus pentasulfide in a stoichiometric amount (Scheme 2) [10, 11].

Scheme 2.
Cook-Heilbron Synthesis: A 2, 4-disubstituted 5 aminothiazole derivative was made when α-aminonitrile and dithioacids/esters of dithioacids, carbon disulfide, and carbon oxysulfide interacted (Scheme 3) [12, 13].

Scheme 3.
Bromoacetophenone and thiourea derivatives are used in the microwave-assisted, alumina-supported synthesis of 2-aminothiazole in dichloromethane (Scheme 4) [14].

Scheme 4.
Acetophenone was heated in water for seven minutes after being microwave-heated with iodine and thiourea for five minutes, results in the 2-amino-4-phenyl thiazole (Scheme 5) [15].

Scheme 5.
Condensation reaction involved copper, oximes, anhydrides, and potassium thiocyanate (Scheme 6) [16].

Scheme 6.
Various transition metals are combined with vinyl azide and potassium thiocyanate to produce 4-substituted 2-aminothiazole (Scheme 7) [17].

Scheme 7.
In the presence of alcohol, cyclic ketones or aryl aldehydes directly interacted with thiosemicarbazide, and the resulting thiosemicarbazones, and then reacted with a-halogenoketones to produce derivatives with a 4-substituted thiazole ring (Scheme 8) [18].

Scheme 8.
Thiocarbohydrazide, aldehyde, and phenacyl bromide were combined to create a thiazole analogue under microwave irradiation in ethanol while also containing the catalytic quantity of ethanoic acid (Scheme 9) [19].

Scheme 9.
Thiosemicarbazones and α-bromoketones are used in the microwave-induced synthesis of thiazoles (Scheme 10) [20].

Scheme 10.
Under microwave irradiation, thiazoles are produced from thiosemicarbazide, ketones, and 2-bromoacetophenone (Scheme 11) [21].

Scheme 11.
- Antimicrobial Activity of Thiazole Derivatives
The phenomena of bacterial resistance increasing have encouraged research towards the development of antimicrobial drugs [22]. The mechanism of antibacterial activity can be used to classify anticipations. Inhibitors of the formation of cell walls, cell membrane depolarizers, inhibitors of protein and nucleic acid synthesis, and inhibitors of metabolic pathways in bacteria are the primary categories [23].
3.1. Antibacterial activity based on the mechanism of action
Antibacterial activity can be demonstrated using the following examples based on the mechanism of action.
FabH inhibitory activity
Via catalysing the condensation of acetyl-CoA with a malonyl-acyl carrier protein to create -ketoacyl-ACP and participating in the feedback regulation of FAS by product inhibition, β-ketoacyl-acyl carrier protein synthase III (FabH) regulates the initial stage of FAS II. In a series of compounds created by Jing-Ran Li et al., compound 35 demonstrated superior antibacterial and Escherichia coli FabH inhibiting activities with a MIC of 1.56e 6.25 mg/mL (Figure 1) [24].

Figure 1.
The compounds 36a and 36b were designed by Peng-Cheng Lv, Kai-Rui Wang, Ying Yang, Wen-Jun Mao, Jin Chen, Jing Xiong, and Hai-Liang Zhu as new thiazole derivatives as effective FabH inhibitors. The compounds 36a and 36b were further placed into the E. coli FabH active site using docking simulation to determine the likely binding configuration, and the results confirmed that the two substances were effective inhibitors of E. coli FabH (Figure 2) [25].

Figure 2.
DNA gyrase inhibitory activity
DNA replication and bacterial DNA supercoiling and uncoiling are both carried out by the enzyme known as DNA gyrase. A unique family of substituted 4,6-dimethyl-2-oxo-1-(thiazol-2-ylamino)-1,2-dihydropyridine was created by Rizk E. Khidre and Ibrahim Ali M. Radini as derivatives 37a, 37b, 37c, 37d, and 37e of 3-carbonitrile. Utilizing in silico molecular docking simulation, the potential DNA gyrase inhibitory activity was investigated. The dock score ranges for novel thiazoles are -6.4 to -9.2 kcal/mol. Compound 37c had strong antibacterial activity with MIC values between 93.7 and 46.9 g/mL, as well as strong antifungal activity with MIC values between 7.8 and 5.8 g/M (Figure 3) [26].

Figure 3.
Ronkin et al. performed a high-throughput screen with the aim of obtaining specific inhibitors of GyrB, which is noticed as an interesting target to win over the cross-resistance to quinolones. Studies on pharmacokinetics and bioavailability focus on the AZD5099 (38) suitability for both parenteral and oral administration
To find particular GyrB inhibitors, Ronkin et al. carried out a high-throughput screen. GyrB has been identified as an intriguing target to overcome the cross-resistance to quinolones. The AZD5099 (38) appropriateness for parenteral and oral administration is the main focus of studies on pharmacokinetics and bioavailability (Figure 4) [27].

Figure 4.
FtsZ inhibitory activity
Prokaryotic cell division protein FtsZ is thought to be a target because it prevents bacterial cytokinesis. From benzothiazolidine derivatives, Ying Li, Ning Sun, and others created a range of thiazole-quinolinium derivatives containing aliphatic amino and/or styrene substituents. In bacterial cells, 39a and 39b induce FtsZ polymerization, according to biochemical experiments (Figure 5). Their primary antibacterial mechanism may involve blocking this crucial phase of bacterial cell division. MRSA and VRE were effectively combated by 5a and 5b's robust antibacterial activity, and low MIC values of 1-8 mg mL and 2-16 mg mL were attained [28].

Figure 5.
In the study by Ning Sun et al., cell-based screening led to the discovery of a novel small molecule 40 with robust antibacterial action and good bacterial cell division suppression (Figure 6). The substance has a wide range of bactericidal activities. The results from both in vitro and in vivo experiments suggested that this substance disturbs the dynamic assembly of the FtsZ protein and Z-ring formation by inducing FtsZ polymerization [29].

Figure 6.
MurB inhibitors
The NADPH-dependent UDP-N acetylenolpyruvylglucosamine reductase known as MurB. plays a crucial part in the manufacture of bacterial peptidoglycan in its second stage. The capability of the 4-thiazolidinone scaffold in inhibiting this enzyme was described by Andres et al. in 2000. They discovered the substance with the highest potency, which has an IC50 value of 7.7 μM and is displayed as follow in Figure 7 [30].

Figure 7.
Tryptophanyl-trna synthetase inhibitors
The aminoacyl-tRNA synthetases are enzymes that covalently join an amino acid to its corresponding tRNA to perform the aminoacylation process. Compound 42 in a series of thiazolin-4-one compounds disclosed by Stana et al. is more efficient against gram-positive bacteria (Figure 8) [31].

Figure 8.
- Micellenous Antimicrobial Activity of Thiazole Derivatives
A unique series of 4-(4-bromophenyl)-thiazol-2-amine derivatives were synthesised by Deepika Sharma et al., and their molecular structures were validated by physicochemical and spectral properties. According to the antimicrobial activity results, compounds 43a, 43b, 43c, and 43d showed potential antimicrobial activity that is comparable to fluconazole and the antibacterial norfloxacin (antifungal).
The discovered compounds 43a shown promising antibacterial activity in vitro against S. aureus and E. coli, respectively (MIC sa and MIC ec = 16.1 µM). Strong antibacterial activity was shown by compound 43c (MIC bs = 28.8 µM) against B.subtilis. According to antifungal activity results, compound 43b (MIC a = 16.2 µM) was determined to be the most effective against A. niger and compound 43d (MIC ca = 15.3 µM) revealed substantial antifungal activity against C. albicans (Figure 9) [32, 33].

Figure 9.
Saima Ejaz et al. created 2-aminothiazole derivatives, and at concentrations of 375 g/mL and 250 g/mL, respectively, 44a and 44b shown substantial antibacterial capability against the gram-negative Pseudomonas aeruginosa and the gram-positive Staphylococcus epidermidis. With a 21.0 mm zone of inhibition, compound 43a had the strongest antifungal potential against Candida glabrata (ATCC 62934). Compared with the reference medicine, nystatin, which has a zone of inhibition of 19.1 mm and exhibits less antifungal potential. The compound 44a-induced zone of inhibition for Candida albicans (ATCC 60387) was 20.0 mm. the substances that have docked with the target enzyme UDP-N-acetylmuramate/L-alanine ligase. The maximum binding affinity was demonstrated by compound 44b (7.6 kcal/mol) (Figure 10) [34, 35].

Figure 10.
Sulfathiazole, a derivative of the thiazole and 2-aminothiazole, has excellent antibacterial, antifungal, and antiviral properties. As an antibacterial agent, sulfathiazole is described as a short-acting sulfa medication, as illustrated in the Figure 11 as compound 45 [36].
The antifungal action of thiazole and its derivatives appears to be effective against many forms of candidiasis. The most effective thiazole derivatives have antifungal activity against all Candida strains that is comparable to ketoconazole and fluconazole, according to the results of the antifungal screening [37].
Derivatives with polyoxygenated phenyl modules exhibit encouraging anti-fungal action; abafungia agent is an example of this type of activity and is illustrated as compound 46 in the Figure 11 [38].
Thiazole derivatives (47) have been shown to have strong antiviral properties against various viruses, including YFV, SARS, RSV, HCV, HRV, VZV, TMV, CVB, FMDV, and influenza virus. Ritonavir is an antiviral drug that functions as a protease inhibitor enzyme and can be used to treat HIV/AIDS [39].

Figure 11.
2, 3, 5-Trichlorobenzene carbothioamide was condensed with phenacyl bromide or dichloroacetone in a study by Karegoudar et al. to produce 45 or 46 antibacterial agents. Moreover, the 2, 3, 5-trichlorobenzaldehyde thiosemicarbazone and phenacyl bromides reaction led to the creation of the (phenylidenehydrazino)-1,3-thiazole derivative 47 (Figure 12). According to their antimicrobial susceptibility testing, some of the compounds had strong antibacterial activity (MIC values equal to ciprofloxacin's standard dose of ¼6.25 mg/mL). The strongest antifungal action was demonstrated by compounds containing 3-pyridyl, biphenyl, and 4-mercaptopyrazolopyrimidine (MIC values ¼ 6.25 mg/mL) [40, 41].
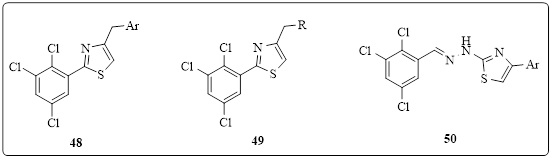
Figure 12.
New 5-hetarylthiazole compounds were created in 2018 by Bondock et al. In vitro antibacterial activity against a few human pathogenic microorganisms was tested on the produced target. The obtained data clearly demonstrate that compounds 51a, 51b, and 51c exhibit higher antibacterial activity with pyrazoline and thiazole side groups (Figure 13) [42].

Figure 13.
A novel antibacterial molecule 52 was created by Santosh et al. and it had a triazole moiety on the side chain of thiazole core (Figure 14). In tests with P. aeruginosa, E. coli, S. aureus, and B. subtilis, this substance demonstrated effective antibacterial action. The main difference between other hybrid compounds and -CH3, -NO2, and -F derivatives was their wide range of activity [43].

Figure 14.
A set of eight thiazoles based on two amino pyrimidines 53a and N-phenylpyrazolines 53b were synthesised and their antibacterial activity was screened by Liaras et al. Two compounds with thiazole and pyrimidine groups showed superior activity (Figure 15). The SAR tests on the thiazole-pyrazoline hybrids showed that the antibacterial action is enhanced by the presence of a phenyl ring. The activity is reduced by the methylamino group. While methyl and fluoro groups lowered activity, the para-substituted methoxy, chloro, and nitro groups modestly increased activity when compared with the methylamino group [45, 46].

Figure 15.
The antibacterial activity studies and syntheses of a novel compound 54 with a thiazole, coumarin, and pyrazole ring all in one molecule were published by Chandak et al. Bacterial strains of S. aureus, B. subtilis, E. coli, and P. aeruginosa were used in the investigations (Figure 16). Results revealed that the chemicals are ineffective against gram-negative bacteria but only moderately efficient against gram-positive bacteria [47].
By combining 2-bromo-4-methoxyacetophenone and 2-acetylpyridine thiosemicarbazone, Bera et al. created pyridinyl thiazole ligand carrying hydrazone moiety 55 (Figure 16). By treating this ligand with cobalt precursor, they also created a cobalt complex. gram-positive bacteria like Bacillus subtilis, Streptococcus fecalis, and Staphylococcus aureus as well as gram-negative bacteria like Pseudomonas aeruginosa, Salmonella typhi, Escherichia coli, Klebsiella pneumonia, and Proteus vulgaris were tested for antibacterial properties of both the ligand and the complex. According to the findings, ligand 55 had MIC values of 200 µg/mL for E. coli and 50 µg/mL for S. Typhi, respectively, and had the best antibacterial action against these bacteria. The ligand becomes more soluble when pyridinium ions are present, which improves the ligand's capacity to penetrate cells and its capacity to bind to cells [48, 49].

Figure 16.
The hydrazinyl thiazole derivatives 56a, 56b, and 56c created by Abeer M. El-Naggar et al. have stronger antibacterial and antifungal effects on E. coli, P. aeuroginosa, S. aureus, B. subtilis, C. albicans, and A. flavus. Derivatization may be continued to produce more powerful and selective agents (Figure 17) [50, 51].

Figure 17.
Compound 57 was created by Armelle T. M. Baveng et al. and demonstrated the best activity with detectable MIC values against 14/16 (87.5%) bacterial strains (Figure 18). In addition, this substance demonstrated greater efficacy than chloramphenicol against E. aerogenes EA289, EA27, and K. pneumonia KP63 [52, 53].

Figure 18.
In their study, Govindasami Periyasami et al. describe a reliable and simple method to synthesize synthesising dispiro-3-phenylpyrrolothiazole hybrids with broad selectivity using a one-pot 1,3-dipolar cycloaddition reaction with environmentally friendly supported solvents and evaluating the biological activities of the resulting compounds. The produced compounds responded to commonly occurring uropathogens, and 58a, 58b, and 58c demonstrated extremely proficient actions (Figure 19) [54-56].

Figure 19.
Fitsum Lemilemu et al. created thiazole-based Schiff base compounds, of which 59 exhibit favourable activities against gram-negative E. coli (14.40 ± 0.04 mm) and gram-positive S. aureus (15.00 ± 0.01 mm) at 200 g/mL in comparison to amoxicillin (18.00 ± 0.01 mm and 17.00 ± 0.04, respectively) [57-59].

Figure 20.
Victor Kartsev et al. in their work to synthesise and to conduct antimicrobial activity of heteroaryl thiazole derivatives they come into a conclusion that compounds 60 and 61 shows best antimicrobial activity against E.coli and B. cereus and S. Typhimurium, respectively (Figure 21). Docking studies were further performed and that indicates a potential role for MurB suppression in the antibacterial action of the investigated chemicals [60-62].

Figure 21.
To find new antifungal agents, a unique set of fifteen hydrazine-thiazole derivatives were created by Cleudiomar Inácio Lino et al. and tested in vitro on six therapeutically significant Candida, Cryptococcus, and Paracoccidioides brasiliensis species. With minimum inhibitory concentration (MIC) values varying from 0.45 to 31.2 M and antifungal activity that was at least as potent as or more potent than the drugs fluconazole and amphotericin B, eight substances demonstrated potential antifungal activity [63-65].
Khare, J. Sharma, and A. Sharm by using well diffusion techniques and doses of 9–10 and 1-2 mg/mL, the synthesized compounds 62 and 63 were tested for their antibacterial activity against gram positive Lactobacillus bulgaris and Streptococcus mitis and gram negative Yersinia (Figure 22). The blocking zones were compared to the common medication Ofloxacin. The manufactured substances displayed average action [66-68].

Figure 22.
12 Different imidazo[2,1-b] thiazole derivatives were created and synthesized in an effort to discover new antiviral and anti-tubercular drugs in the study carried out by Elif Gürsoy et al. The substances underwent testing for their antiviral and antitubercular properties. In CRFK cell cultures, the antiviral activity and cytotoxicity of the compounds were examined against the feline corona and feline herpes viruses. When compared with HHA, UDA, and ganciclovir standards, compound 64 was found to be extremely efficient (Figure 23)[69, 70].

Figure 23.
- Conclusion
Thiazoles are highly desired and biologically active. According to the aforementioned literature review, such thiazoles can be produced simply from a wide range of starting materials. The pressing demand for innovative and potent antimicrobial drugs stems from the high mortality rate of antibiotic resistance. According to the investigations, microwave irradiation of acetophenone with iodine and thiourea appears to be the most practical approach for the production of thiazole derivatives. It was believed that these changes to the thiazole moiety indicated beneficial biological activity and would show more in the future. Thiazole compounds were compiled for this review's anti-microbial activity. A study should be conducted to examine much more effects of thiazole's anti-disease.
Acknowledgment
I wish to express my sincere thanks and gratitude to Professor Dr. Arun kumar R who guided me in this work.
Orcid:
S. Swathykrishna: https://orcid.org/0000-0002-8815-0235
Amrithanjali: https://orcid.org/0009-0004-0984-248X
Gisna Shaji: https://orcid.org/0009-0001-8150-5910
Arun Kumar R.: https://orcid.org/0000-0001-5418-6136
Citation: C. S. Swathykrishna *, G. Amrithanjali, G. Shaji, A. Kumar R., Antimicrobial Activity and Synthesis of Thiazole Derivatives: A Recent Update. J. Chem. Rev., 2023, 5(3), 221-240.


