Document Type : Review Article
Authors
Research Scholar, Department of Chemistry, Christ University, Bangalore, India
Abstract
Superabsorbent hydrogels are hydrophilic polymer units that can absorb water and organic fluids into the three-dimensional network and mimic biological cells when swollen. Hydrogels are categorized as natural, synthetic, and hybrid, depending on their constituent polymer. The novel green synthesis includes the combination of natural polymers with synthetic ones to produce eco-friendly Hydrogels. The networks are established using crosslinkers formed chemically as covalent bonds or ionic bonds and physically if intermolecular forces are involved. Starch and cellulose are naturally occurring biopolymers that make significant applications for hydrogel production. This article reviews hydrogel, its properties, classification, synthesis mechanism, and application in various sectors using starch and cellulose as copolymers. Due to the high range of availability, nontoxic nature, and biodegradability, starch and cellulose-based hydrogels find high regard in the present research era. The biopolymers beneficiation can result in the evolution of economic and sustainable methods for transforming this natural biopolymer into utilitarian organic products.
Graphical Abstract
Keywords
Main Subjects
- Introduction
Hydrogels are 3-D structural materials that can intake large amounts of water including biological fluids and do not dissolve due to their physical or chemical entanglement in the network [1, 2]. The water absorption capacity of hydrogel is due to the presence of its functional groups such as -NH2, -CONH2, -COOH, and so on [3]. The hydrophilic polymer units of the hydrogel can absorb water from an arbitrary weight of 10% to a thousand times its dry weight. If the water is composed of more than 95% of the total weight, the hydrogels are known as superabsorbent [4, 5].
Many hydrogels are synthesized with the intention of specific applications like transdermal drug delivery, wound dressing, and contact lenses, in the soil for water absorption, and retention, and for the controlled release of fertilizers [2]. The most commonly used synthesis method is light-induced photopolymerization which requires a photoinitiator which is a light-sensitive molecule that upon irradiation with UV, visible or IR can produce active species. The crosslinker and the functional group binding to the polymer can create temporal and special control over reactions [6].
The chloroplast of a leaf contains starch, which is the most common polysaccharide storage in plants and appears as granules. A typical term for the starch found in seeds, legumes, and tubers is amyloplast. In its unprocessed state, starch is a tasteless, white powder. A high number of glucose units make up the structure of starch, which is joined together by glycoside bonds [7].
As illustrated in the Figure 1 two key structural elements of starch are amylose and amylopectin. Amylose is linear or just slightly branched, while amylopectin is heavily branched. 30% of the mass of starch granules is thought to be crystalline, and 70% is thought to be amorphous. The majority of amylose and a sizeable portion of amylopectin are found in the amorphous regions. The crystalline area predominantly comprises amylopectin. The amount of amylase present and gelatinization temperature are the two key process variables influencing gel formation [8]. The advantages of using starch are its availability, low cost, renewable, biocompatibility biodegradability, and nontoxicity [9]. Starch's intermolecular bonds are broken during the gelation process when water and heat are present. A more rigid structure can be created by alkaline gelatinization than by heating-induced gelatinization in the hydrogel. By boosting the starch's attraction for water, the hydroxyl groups of the glucose units in the starch structure get ionized at high pH [10].

Figure 1. Structure of starch molecule
As the primary component of plants and natural fibers like cotton and linen, cellulose is a naturally occurring glucose polymer. Some bacteria, like Acetobacter xylinum, can also produce cellulose [11]. Cellulose is composed of a d-glucose unit and a C4-OH group at one end, which is the non-reducing end. The terminating group is a C1-OH group, which is the reducing end with an aldehyde structure (Figure 2) [12].

Figure 2. Structure of cellulose
Even in extreme thermal settings, cellulose has strong resistance to the UV radiation. In particular, cellulose and its derivatives are widely sought after for the food industry, medicine, agriculture, green energy production, and textile uses due to their mechanical strength, biocompatibility, and environmental sustainability [13].
The incorporation of biopolymers like starch and cellulose improves the biodegradable performance of the hydrogel. In natural conditions, most biopolymer hydrogels are converted into CO2, H2O, and biomass under the enzymatic action of the microbes [14]. Water treatment methods that are both affordable and environmentally friendly use starch derivative adsorbents (SDAs) to remove or adsorb aqueous heavy metal ions (AHMIs) from water [15]. The biocatalyst amylase enzyme is utilized to break down the starch amylopectin. Kirchoff investigated the amylolytic action of starch-degrading enzyme in 1811, and amylose was shown to be a particular enzyme for this activity [16]. The Ag NPs loading in hydrogel networks increases the anti-bacterial activity [17].
This report reviews the benefits of using starch and cellulose-based hydrogel in various applications. Since these biopolymers are large sources of polysaccharides, their sustainable conversion can cause the production of high-value materials and the development of cost-effective technologies for converting waste into the useful products. The classification, swelling mechanisms, and synthesis techniques of hydrogels are further reviewed.
- Classification of Hydrogel
Based on the source, hydrogels are categorized as natural and synthetic hydrogels [1]. Hydrogels are grouped into homopolymer-polymer, multipolymer, or interpenetrating polymers depending on their polymer constituents. Homopolymers are derived from the same type of monomers, and co-polymers constitute two or more types of monomers with one hydrophilic moiety. Interpenetrating polymers are a salient class of hydrogels that are made of two self-reliable crosslinked synthetic or natural polymer units [18].
Another method to classify hydrogels is depending upon the cross-linkage established either by physical or chemical hydrogels. Chemical hydrogels are again grouped on the basis of covalent bonds within the matrix [19]. A covalent bond determines the degree of water absorption capacity of hydrogel which in turn depends on the hydrophilic nature of polymer and the range of reticulation taking place in the matrix. Physical hydrogels are formed from molecular entanglements, physical interaction, ionic bonding, or hydrogen bonding, and these hydrogels are also known as temporal hydrogels due to their reversible nature. Hydrogels are categorized based on the charge as cationic, anionic, and neutral [20]. The reversibility can occur due to the application of some forces or environmental changes such as pH, temperature, pressure, and electric and magnetic fields [2]. Depending on the configuration and physical appearance hydrogels are classified as crystalline, semicrystalline, and amorphous structures [21]. Hydrogels are further classified based on their intelligence to respond to environmental stimuli such as pH, light, temperature, electric fields, pressure, magnetic field, solvent composition, and sound (Figure 3) [18].

Figure 3. Classification of hydrogel
- Swelling Mechanism of Hydrogel
Swelling is the most important feature of hydrogel technology. The hydrogel effect on various applications is studied through swelling and deswelling test [22]. When a hydrogel is placed in the water, the water molecules which enter initially hydrate the most hydrophilic groups, resulting in “primary bound water.” As the hydrophilic groups are hydrated, the polymer enlarges by swelling and exhibits out the hydrophobic groups with water molecules, forming hydrophobically bound water which is called “secondary bound water.” Primary and secondary bound water are combined and called “total bound water.” Once the hydrophobic and hydrophilic ends are entangled with water, the network imbibes additional water due to the osmotic forces. The additional swelling water after the polar, hydrophobic, and ionic groups are saturated with bound water is called “bulk water” or “free water” that can fill the space between the polymeric network chains and the center of large pores and voids (Figure 4) [23, 24].

Figure 4. Swelling mechanism of hydrogel
- Synthesis Mechanism of Hydrogel
The mechanism used to carry out crosslinking and polymerization determines the hydrogel synthesis process [25]. The network inside the matrix and the presence of bonding within the polymer chains cause cross-linking, which inhibits the dissolution of polymer units prior to practical use [26]. As a result, changes in the physiochemical characteristics of the hydrogels are centered on how crosslinked and crystalline they are. The flow and deformation of the polymer, the glass transition temperature, and the increase in crosslinked points are some of the alterations [27]. Other changes include a decrease in elasticity, viscosity, and solubility. The covalent or secondary connecting points of many chains are referred to as crosslinks [28]. It can be a junction formed by crystallites or subsequent interactions, or it might be a covalent link-a tiny chemical connection between carbon atoms. The presence of crosslinks in a hydrogel's structure is strongly correlated with its integrity [29]. Thus, the crosslinking network of different hydrogels is used to distinguish them. Crosslinking can be done physically or chemically to create hydrogels.
4.1 Physical crosslinking
The polymers containing polar functional groups like hydroxyl, acylamino, and carboxyl groups, crosslinking via hydrogen bonding is conceivable [30]. Hydrogen bonding can happen with other polymers containing electron-deficient hydrogen atoms when carboxyl groups protonate [31]. Physical linking takes place through the coacervation process which contains a coacervation phase and an equilibrium phase [32]. The Coacervation phase contains a high amount of collides, whereas the equilibrium phase contains very little amount of collides [33]. A simple coacervation involves a single polymer, but there should be at least two oppositely charged polyelectrolytes for complex coacervation [34]. Hydrogels can be further formed through heating or cooling. This is possible, especially with polymers that have helices, helix-like formations, and junction zones present in their structure [35, 36]. Carrageenan-gelatin hydrogels are prepared by mixing hot stock solutions of each precursor at 40 °C in different weight ratios [37]. The crystallites in a polymer chain can act as physical crosslink sites in the network, leading to the formation of a hydrogel [29]. Generally, Physical cross-linking is the process by which weak physical interactions create a binding between polymer chains in the solvent condition (Figure 5). Coordination bonds, hydrogen bonds, ionic contacts, and Van der Waals interactions are some of these interactions.

Figure 5. Physical crosslinking
4.2 Chemical crosslinking
4.2.1 Free radical polymerization
A chain-growth polymerization process called free radical polymerization (FRP) uses initiators to produce free radicals by either homolytic dissociation or redox reaction (Figure 6) [38]. In free-radical polymerizations, the active site at the chain end is renewed when a monomer molecule is added to an active chain end. Monomers' carbon-carbon double bonds are typically where free radicals can initiate chain propagation. The chain then breaks when the radicals that are propagating react by combining, distorting, and transferring. With this, the FRP method is the most popular chemical crosslinking method for making hydrogels.

Figure 6. Free radical polymerization
4.2.2. Photopolymerization
The in situ creation of crosslinked networks is made possible by photopolymerization [39]. It offers a special method for quickly and precisely manufacturing gels [40]. This method involves the use of UV-Visible light to interact with light-sensitive substances, known as photoinitiators, and FRP to transform a liquid monomer or macromer into a hydrogel (Figure 7). The mechanism for photoinitiation depends on the photolysis processes: photo-cleavage, hydrogen abstraction, and cationic photopolymerization.

Figure 7. Photopolymerization
4.2.3 Crosslinking induced by enzymatic reactions
Enzymatic reactions allow the formation of strong covalent bond and rapid gelation in less time under physiological conditions (Figure 8) [29]. This reaction takes place under normal temperatures. Apart from fast gelation, enzymatic crosslinking gives adjustable mechanical properties and controllable degradation copolymerization [39].

Figure 8. Enzymatic crosslinking
4.2.4 Crosslinking by click chemistry
Barry Sharpless came up with the term "click chemistry," which refers to a class of reactions that are quick, adaptable, purifiable, and have high product yields [41]. The procedure was developed to resemble the spontaneous aldol condensation of raw materials. The C-C bonds in the backbones of natural goods often serve as a link [42]. For its synthetic reproduction, a strong thermodynamic driving force is needed. This issue is addressed by click reactions, which pair a C atom with plentiful heteroatom X found in polysaccharides. As a result, to link proteins, nucleic acids, and/or polysaccharides, a C-X-C bridge is created as opposed to a C-C bond. The hydrogel crosslinking based on click chemistry includes the following: Diels–Alder, Schiff base, oxime, Michael-type addition, and boronate ester [43].
Starch and imide can react using click chemistry, which is a class of reactions that are highly efficient, selective, and often occur under mild conditions (Figure 9). One example of a click reaction that can be used to modify starch with imide is the copper-catalyzed azide-alkyne cycloaddition (CuAAC) reaction [44].

Figure 9. Click reaction
4.2.5. Grafting
Graft polymerization is an adaptable method for incorporating desired functional groups into the polymer backbone, creating a new hydrogel with specific properties (Figure 10) [45]. Grafting is the process of creating active sites by removing hydrogen atoms from the polymer backbone. To create a graft copolymer, this creates macroradicals to which desirable monomers can attach [46, 47]. The molecular makeup, length, and quantity of the side chains determine the characteristics of the graft copolymers that form [48]. Grafting can be accomplished by enzymatic, chemical, radiation, photochemical, and chemical techniques.
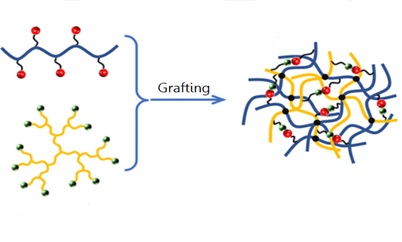
Figure 10. Graft polymerization
- Significance of Starch and Cellulose Hydrogels
Synthetic polymers exhibit certain exceptional mechanical, physical, and chemical properties, due to which they are frequently employed to create the hydrogels that are currently sold on the market [49]. In spite of their wide application, they are difficult to biodegrade, non-renewable, and unsustainable in terms of production. For these reasons, scientists are paying increased attention to hydrogels made from naturally occurring polysaccharides like starch and cellulose [50, 51].
Plants are a rich source of starch polysaccharides, and the chloroplast in green leaves and the amyloplast in the tubers and seeds are particularly rich in granular starch. The main production source of commercial starch are potatoes, wheat, corn, and rice [52, 53]. Starch is converted into gelatin primarily through three stages of hydration and plasticization. Granules of hydrophilic starch absorb water and expand in the initial stage. When the starch is dissolved in water and turns into gelatin in the next step, the granule structure is destroyed. The development of the starch hydrogel network by chilling and aging and the reorganization of polysaccharide structure make up the last phase. This phase is also known as the retrogradation phase [54, 55]. There are many chemical techniques that can be used to create starch-based hydrogels, including starch grafting and etherification. The -OH groups from the starch molecule are replaced in etherified starches with various ether groups, such as carboxymethyl starch. In contrast, the grafted starch approach creates hydrogels by attaching various vinyl monomers to starch [56, 57].
Though it is highly eco-friendly to use starch, the low surface area, the requirement for chemical derivatization to increase the sorption capacity, and the necessity for low durability, among other shortcomings, are some downsides of starch-based hydrogels. The carbon-carbon backbone of starch was used to give vinyl polymers a biodegradable structure. The usage of starch results in an increase in overall surface area, which makes microorganisms more likely to attack [58]. The biodegradability increased significantly by this method, although it was not entirely environmentally benign. To mix with starch, aliphatic polyesters, polyvinyl alcohol (PVA), and biopolymers are frequently used. The primary goal of this blending is to increase biodegradability, while retaining cost-effectiveness and other qualities. Polylactic acid, or PLA, is a good polymer in terms of biodegradability and is extensively used in the biomedical industry. High strength, modulus, and biocompatibility are some of PLA's best qualities [59].
Amylase is an enzyme that catalyzes the breakdown of starch into smaller molecules such as maltose and glucose. When amylase is added to a starch hydrogel, it will initiate the process of starch digestion (Figure 11). When hydrogen peroxide is applied to the surface of a starch hydrogel, the reaction may primarily occur at the interface between two substances. This may result in the formation of bubbles of carbon dioxide gas on the hydrogel surface, which can cause it to expand and become more porous.

Figure 11. Enzyme catalyzed reactions in starch hydrogel
The extensive availability of cellulose (as a natural biomaterial) in the form of live terrestrial biomass suited for various industrial uses is one of the numerous practical benefits of using it in hydrogel compositions [60]. Each hydro-glucopyranose unit in cellulose molecules contains three alcoholic hydroxyl groups, allowing for feasible chemical changes to be made to these hydroxyl groups. There are 30-36 cellulose chains linked laterally through hydrogen bonding in crystalline regions, where a single cellulose chain often traverses across both crystalline and amorphous regions. Methylcellulose (MC) [61], hydroxyethyl cellulose (HEC) [62], hydroxypropyl cellulose (HPC) [63], hydroxypropyl methylcellulose (HPMC) [64], and carboxymethyl cellulose sodium (CMC Na) [65] are a few cellulose derivatives that have been produced to create hydrogels. These derivatives are understood to be cellulose derivatives that are water-soluble.
An efficient and environmentally beneficial coating material for slow-release mono ammonium phosphate fertilizer (MAP) is a biodegradable all-cellulose composite hydrogel. The sodium carboxymethyl cellulose/hydroxyethyl cellulose mixture used to create the biodegradable formulation was filled with 5% of spherical regenerated cellulose particles (Figure 12) [66].

Figure 12. Cellulose composite
Methylcellulose has a special property of forming a thermal reversible upon heating and considers a polymer with a lower critical solution temperature [67]. Citric acid is a natural crosslinker. An increase in the crosslinking degree may cause an increase of crosslinking points which prevents the expansion in aquatic environment. The less use of citric acid (5%) would reduce the affinity of coating of water and oxygen used for food preservation and to preserve nutrients susceptible to oxidation [68]. When the HEC degree increases, the solubility level in water will increase. HPMC is a water-soluble polymer that is available in several grades with different viscosities and substitution rates. The microporous and chemical structure of crosslinked HPC resin produces hydrogels with high adsorption capacity of anions. Among the five cellular derivatives, CMC Na remains the favorite raw material for producing hydrogel.
- Structural Properties of Starch and Cellulose Hydrogels
As illustrated in Table 1 starch and cellulose hydrogels are both widely used in various applications due to their unique functional properties. Starch hydrogels have excellent water absorption capacity due to their high hydrophilicity. This property makes them suitable for use as absorbent materials in hygiene products and wound dressings. Starch hydrogels can form a gel when exposed to heat, acid, or enzymatic action. This characteristic makes them useful in food processing and as drug delivery systems. Starch hydrogels can adhere to various surfaces due to their sticky nature. Cellulose hydrogels have excellent mechanical strength and are highly biocompatible causing zero toxic effects. Cellulose hydrogels are transparent, which makes them suitable for use in contact lenses and optical devices. Both cellulose and starch are naturally abundant biopolymers that are feasible for various applications due to their eco-friendly properties. These properties vary due to their structural makeup and natural inbuilt system.
Starch-based and cellulose-based hydrogels have their own limitations, and the best choice will depend on the specific requirements of application. It is important to consider their advantages, disadvantages, and their potential impact on the hydrogel performance before choosing the most appropriate type of hydrogel for a given application.
Table 1. Comparative study between starch and cellulose hydrogel

- Applications of Starch and Cellulose Based Hydrogels
7.1. Agricultural applications
Agriculture is the most important industry which adequately relays on the quantity of water and nutrients. Hydrogels are used in agriculture due to their significant advantage such as: (I) the usage of less amount water for irrigation, (II) the controlled release of fertilizers, and (III) enhancing plant growth and reducing environmental pollution [77]. They can be used for water storage and fertilizer retention and prevents the fertilizer from early leaching and surface runoff. Starch-based hydrogels can enhance bacterial growth and control weeds and harmful microorganisms [78]. Ultimately controlled fertilizer usage is a great use of hydrogel in the farmlands. The SH prepared from starch and poly (methacrylic acid), adding initiator (ammonium persulphate) and crosslinker (N, N-methylene-bis-acrylamide) was used for the slow release of thiram fungicide [79]. SH co-polymers of acrylic acid and acrylamide were used as the membrane of urea for controlled release [80]. Abd El-Mohdy et al. prepared starch hydrogel for the slow release of thiophanate methyl, fluometuron, and trifluralin. The main global issues we face today are water scarcity and environmental pollution which can be solved to a certain extent using hydrogel [81]. The hydrogel system is penetrated by osmosis when the polymers come into contact with water, and as a result, hydrogen atoms react and emerge as positive ions. Along the polymer chain's entire length, this reaction leaves behind a number of negative ions. These repelling negative charges cause the polymer chain to unwind and open up. They also attract water molecules and use hydrogen bonds to bind them (Figure 13) [82].

Figure 13. Swelling mechanism of hydrogel in the soil
The creation of environmentally safe and sustainable slow-release fertilizers is a desirable use for cellulose-based products. The cellulose structure's adaptability and functionalization make it a suitable scaffold for the creation of high-tech agricultural goods [83]. The ability to encapsulate herbicides into the structure of cellulose-based superabsorbent hydrogels allows for effective control over the herbicides release, which has significant economic and sustainability consequences for the agriculture industry. It is one of the most effective methods of weed and insect control in agriculture to prevent any negative environmental effects [84].
7.2. Biomedical applications
The use of hydrogel in drug delivery devices is the most important application and they are already in the use. The primary goal of developing a drug carrier system is to deliver the site-specific medication to the body as effectively and with as few side effects as possible [85]. Due to the fact the drug molecules in these sorts of site-specific medications do not come, in contact with other body tissues and organs, they exhibit increased efficacy and have several distinct and adaptable physical properties that make it particularly helpful to use them in a medication delivery system [86]. Because SHs have a porous surface, they have a strong attraction to liquid environments and are swelled. The crosslinking density can be changed to vary the porosity of Hydrogel. Porosity is a crucial characteristic since it enables drug molecules lost into hydrogels quickly and easily and aids in the drug’s release in the particular system [87]. Reis et al. explored starch-based hydrogels (starch-M hydrogels) with glycidyl methacrylate for the treatment of colon disorders including Crohn’s disease. The acid- responsive medication corticoids were preserved and transported with outstanding behavior by this hydrogel. The studies suggest that hydrogels can be used in drug delivery due to their selective permeability [88]. The nanocellulose carboxymethylation involves the introduction of carboxymethyl groups onto the surface of nano-cellulose fibres (Figure 14).
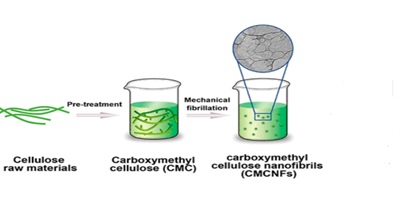
Figure 14. Cellulose nanofibrils
This modification alters the chemical and physical properties of nanocellulose, making it more soluble in water and giving it an improved surface charge. CMNC has a number of potential applications, including in the development of new materials, such as biodegradable films, coatings, and composites. It can be further used as a rheology modifier, thickener, or stabilizer in various industries, including food, cosmetics, and pharmaceuticals.
Due to their exceptional qualities and ecologically friendly characteristics, nitrocellulose-based hydrogels, such as those made of cellulose nanocrystals (CNC), cellulose nanofibrils (CNF), and bacterial cellulose (BC), have a number of advantages as drug carriers. Nanocellulose greatly enhances the hydrogel characteristics and adjusts drug-releasing profile in nanocellulose hydrogels, which have been shown to sustainably deliver diverse types of pharmaceuticals via multiple routes of administration [89]. With the use of ultrasound in biomedicine, sonochemistry provides a quick and environmentally friendly method of material synthesis. The small acoustic bubbles that the sound wave creates as it travels around the room include an amazing facility where matter interacts with other matter at energies as high as 13 eV to cause spectacular chemical reactions. In addition to increase the effectiveness of cellulose extraction from raw materials, ultrasonication has an impact on the hydrogels creation [90].
7.3. Food industry
The fruits and vegetables are getting rotted due to the evaporation of water and the decomposition of components, also due to the breathing of fruits and vegetables even after harvesting. Alternative methods of preserving the fruits for a long time include freezing, low-temperature storage, and CA storage (low oxygen and high carbon dioxide) which stops breathing and prevents evaporation of water and degradation [91]. The packing practice at a low specific low temperature reduces the preservation cost. Water evaporation is prevented and the CA effect is obtained by its preservation in a sealed condition. To manage the CO2 and O2 content, and the humidity for preserving fruits and vegetables like carrots and spinach, T suji et al. coated a low-density polyethylene film with a starch-graft-acrylic acid as a water-absorbing layer and used urethane as a binder [92].
The starch antibacterial film is a type of edible film that can be applied to the surface of fruits to protect them from contamination by bacteria and other microorganisms (Figure 15). The film is typically made from a mixture of starch and other ingredients, such as plasticizers, antimicrobial agents, and other additives. Antibacterial films are biodegradable and can be consumed along with the fruit, reducing waste and environmental impact. Likewise, they can help to maintain the natural appearance and texture of the fruit, which can be important for marketing and consumer acceptance.

Figure 15. Starch antibacterial film
Cellulose-based hydrogels can be utilized in packaged goods including meats, fresh fruits, vegetables, and other high-water-content foods as an active packaging material and as humidity controllers [93]. According to a different study, lactic and acetic acids are safe cleaning chemicals used in the meat business. However, due to their low cost and simple management, solid antimicrobial agents are constantly in demand, particularly in the food business.
Porous antimicrobial hydrogels are created when cellulose and its derivatives are crosslinked enzymatically with organic acids (lactic and acetic acids). AgNPs, ZnO, and CuO nanoparticle-containing CBH films showed more antibacterial efficacy against E. coli than L. monocytogenes. Fresh potatoes can have their shelf life extended using the CBH film [94].
In the food processing sector, nutritional quality and food protection are of the utmost importance. Food quality monitoring can be done quickly, affordably, and non-destructively using hydrogel-based biosensor. A functional hydrogel consisting of D-glucose pentaacetate, silver ions, and agarose is used to assess the formation of biogenic amines (BAs), which signal the freshness of fish. When compared to the other previously described BAs sensors, this hydrogel-based biosensor avoids the pre-synthesis of fluorescence probe, making it more practical and affordable for evaluating the freshness of fish [95]. The hydrogel is also employed to evaluate the toxicity and bacterial toxicity [96]. The accessible hydroxyl groups on the CNC surface, which allow for a high degree of surface-bound fluorophores, may be used by CNC-based hydrogels for the detection of pH changes in meals [97]. In addition, the cholesterol oxidase/CA/carbon nanotubes/biosensor designed for cholesterol detection demonstrated improved performance and excellent sensitivity with a detection limit of 108 M [98]. Promising hydrogel solutions in the food packaging sector include enhanced barrier qualities (gas and moisture), antimicrobial packaging, active and intelligent packaging, nanoparticle additions, shelf-life augmentation, oxidation prevention, and flavor masking [99]. Due to a higher rate of respiration during storage, fresh potatoes are wrapped in plastic containers to prevent fogging, which is the buildup of water on the containers’ surfaces. Both of these effects-the potato becoming green and the effect-is avoided by the integrated hydrogel packaging technique. Furthermore, the ferulic acid-incorporated antioxidant components in CBH films stopped the oxidation of lipids in butter [100].
The primary obstacle of starch hydrogel is retrogradation in the presence of water. This problem can be solved by preparing thermoplastic starch with various plasticizers [101]. Starch films can be created using various procedures, such as term processing or casting techniques [102]. Starch/clay nanocomposite-based biodegradable food packaging materials were created by Avella et al. [103]. The packing displayed good mechanical qualities (modulus and tensile strength). Starch films are made using a variety of starches, including modified, soluble, and pre-gelatinized starches. Starch films have a number of benefits, including being transparent, odorless, biodegradable, non-toxic, and having low oxygen permeability at low humidity [104]. Omega fatty acid-rich oils, such as those found in plants and fish, are known to be chemically unstable and prone to oxidation and deterioration. Encapsulation with starch or starch composite is a widely used technique to minimize fatty acid oxidation and the disagreeable flavour of oxidation products [105]. Systems based on starch can be further used to extend the shelf life of food goods. For instance, lyophilized microspheres with an average particle size of 1.01 m were created utilizing fresh tiger nut milk as the core material and a combination of inulin and tiger nut starch that has undergone OSA modification [106]. Lei et al. [107] successfully generated olive oil-loaded porous particles by plating and compared the oxidation stabilities of free and encapsulated olive oils using porous purple sweet potato starch created through enzymatic modification. Due to their effective film-forming abilities, acetylated maize starch and maltodextrin could enclose heat-sensitive anthocyanins during a spray-drying procedure.
- Conclusion
The greatest reserves, most extensively dispersed, and most readily degradable substances in nature are cellulose and starch. Therefore, the advancement of green chemistry is significantly impacted by the creation of functional cellulose and starch materials. Hydrophilicity and high biocompatibility are two functional traits of cellulose and starch that make them suitable carriers for various industries. The features, preparation techniques, classification, properties, and uses of hydrogel in many domains are covered in detail along with a summary of the research so far on cellulose and starch-based hydrogel materials. In light of the aforementioned research, it is determined that cellulose and starch hydrogels exhibit the following key benefits when used as carriers. First, the cellulose hydrogels' three-dimensional network raises the specific surface area, which significantly enhances the adsorption performance. Second, cellulose's negative ions and a sizable amount of hydroxyl groups can boost the material's capability for adhesion as well as its stability and swelling.
Even though the study of hydrogels made of cellulose and starch is currently ongoing, significant problems remain (1). Radiation pre-treatment offers the advantages of being environmentally friendly and highly effective, but comparatively, little research has been done on this technique (2). Whether or not the bio-application will have an impact on a hydrogel carrier's mechanical strength, more study is required (3). The majority of hydrogel applications are concentrated in the environmental sector, with very few established in the energy sector. There has not been any investigation of medical practices like photodynamic therapy. Future studies can thus concentrate on this issue and perfectly enhance its biocompatibility to permit its use in the medical industry.
Acknowledgment
We are grateful to Christ University, Bangalore for the timely help and encouragement to publish this review paper.
Orcid:
Manju Manuel:
https://orcid.org/0000-0003-3738-440X
Anvil Jennifer:
https://orcid.org/0000-0002-6807-5397
Citation: M. Manuel*, A. Jennifer, A Review on Starch and Cellulose-Enhanced Superabsorbent Hydrogel. J. Chem. Rev., 2023, 5(2), 183-203.


