Document Type : Review Article
Authors
- Ihssin Abubaker Abdalsamed 1
- Ibrahim Ali Amar 1
- Abubaker Ahmed Sharif 2
- Masood Abdulsalam Ghnim 3
- Abdusatar Abduallah farouj 4
- Jamal Ali Kawan 5
1 Department of Chemistry, Faculty of Sciences, Sebha University, Sebha Libya
2 Libyan Advanced Centre for Chemical Analysis, Libyan Authority of Science Research, Tripoli, Libya
3 Department of Geology and Environment, Faculty of Sciences, Bani waleed University, Libya
4 Department of Corrosion, Total Oil Company, Tripoli
5 Department of Production, Akakus Oil Company, Tripoli
Abstract
Scale corrosion is the worst type of corrosion compared to other types of corrosion due to the tendency to attack pipes is higher even when the pipes are made of non-metals such as polymers. Scaling can cause technical issues such as equipment and pipe obstruction, as well as significant damage and financial losses.
This review focuses and outlines the types of scale corrosion, scale corrosion control methods such as chemical methods and non-chemical methods (preventing scale formation), scale corrosion removal methods, and scale corrosion monitoring processes. Finally, we provide some perspectives effect of CO2, O2, and H2S on scale corrosion.
Graphical Abstract
Keywords
Main Subjects
1. Introduction
Water is the one thing most important to human life in the world. Scale corrosion has been considered the first fatal destroying agent of water plants such as drinking water distribution systems, cooling water systems, water wells, storage tanks, boiling water systems, water treatment systems, crude oil plants, and industrial plants, causing billions of US dollars in a breakdown of the equipment while time-consuming associated with water leakage. This is the major challenge, and it is the primary reason that many companies and researchers are developing new technological methods to manipulate corrosion problems on a large scale. Corrosion is a degradation phenomenon of a metallic material resulting from the chemical or electrochemical interaction with its environment. Cracking and pitting corrosion can occur when H2S, CO2, O2 and SRB bacteria are present, whereas scale corrosion can occur when CaCO3, CaSO4, BaSO4 and Fe compounds are present The chemistry of iron compounds is substantially more complex than that of other compounds, because iron occurs in water in two oxidation states, Fe2+ (ferrous) and Fe3+ (ferrous) (ferric). Because these two ions produce compounds with the same anions but widely different solubility, it is not easy to anticipate iron compounds' behavior quantitatively. The presence of iron ions in water indicates corrosion. The effect of dissolved gases is as follows: carbon dioxide reacts with iron to form iron carbonate scale, hydrogen sulfide reacts with iron to form iron sulfide, and oxygen reacts with iron to form ferrous hydroxide Fe (OH)2, ferric hydroxide Fe(OH)3, or ferric oxide Fe2O3. Red water is caused by Fe2O3 particles suspended in the water [1]
2. Scales form
The maximum amount of a solute dissolved in a solvent under a given set of physical conditions is called solubility. In aqueous solutions, the chemical species we're interested in are found as ions. Water solubility is low in compounds containing particular combinations of these ions. Water's ability to retain these compounds in solution is limited, and when that limit is reached, the compounds precipitate out of solution as solids (Figure 1).
As a result, if the water contains ions capable of forming restricted-solubility compounds, and the physical circumstances or water composition change, the solubility falls below the concentrations present, solid materials that may form scale will precipitate. Solid precipitates can float in water or form a scale on a smooth surface, such as a pipe wall. Filtration of suspended particles from water may create clogging of the formation.
A solid scale may emerge on the formation face. Neither alternative is appealing. The difficulty of removing a clog varies depending on the type of clogging that has occurred. Scale can clog injection and flow lines, as well as tubing strings, obstructing flow. It causes pump wear or obstruction, and additional rod strains when it accumulates on sucker rods. Fire tubes in all heaters break early when scale buildup causes overheating. Scale deposits commonly exacerbate corrosion.
Finally, a series of manufacturing difficulties can be traced back to water-formed scales. Effective scaling management should be one of any efficient water injection operation [2-4].

Figure 1. The scale corrosion on pipe
2.1 Common scales
Of the many possibilities, only a few water-formed scales are usually discovered in water distribution systems and oilfield waters [5]. These scales, and the key variables that influence their solubility are given below [6].
Table 1. Most common scales
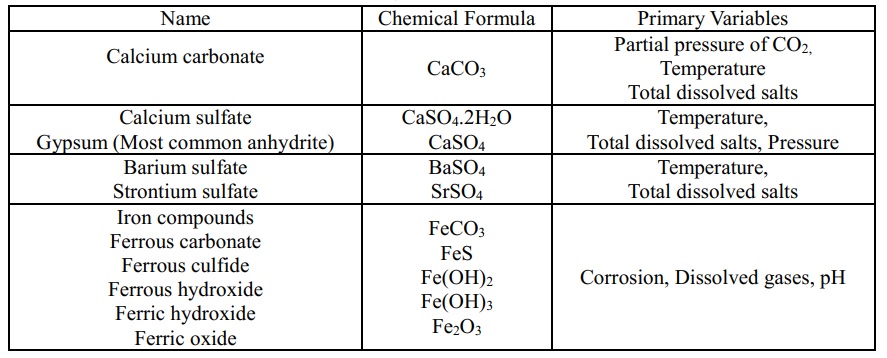
2.2 Calcium carbonate scale
Calcium carbonate scale is created when calcium ions are combined with carbonate or bicarbonate ions in Equation 1 and 2:

![]()
2.2.1 CO2 Effect
The solubility of CaCO3 in water is increased by CO2 pressure. Carbonic acid is formed when carbon dioxide dissolves in water and ionizes according to the following Equation (3-5):

As indicated in Equation 5, only a small percentage of bicarbonate ions breakdown to form H+ and CO3 - -. Under typical conditions, the amount of bicarbonate ions presents far outweighs the carbonate ions. As a result, it is assumed that Equation 2 is the correct expression for calcium carbonate precipitation.
The reaction moves to the left as the concentration of CO2 in the solution rises, resulting in less CaCO3 precipitation. With the addition of CO2, the water likewise gets more acidic (the pH falls).
The partial pressure of CO2 in the gas over the water determines the amount of CO2 that will dissolve in water:
The partial pressure of CO2 = (mole fraction of CO2 in gas) (total pressure)
The partial pressure of CO2 in the gas phase lowers, CO2 comes out of solution, and the pH of the water rises at any point in the system where a pressure drop is taken. This causes CaCO3 precipitation by shifting reaction (2) to the right [7].
2.2.2 pH Effect
The pH of the water and the solubility of calcium carbonate are both affected by the amount of CO2 present. However, it makes no difference what causes the water to be acidic or alkaline. CaCO3 precipitation is less likely at lower pH. Conversely, the higher the pH, the greater the likelihood of precipitation [8].
2.2.3 Temperature effect
In contrast to most materials, calcium carbonate becomes less soluble as the temperature rises. The higher the water temperature, the more likely it is that a CaCO3 scale will form. As a result, if the downhole temperature is high enough, water that is non-scaling at the surface might cause scale formation in the injection well. CaCO3 scale is frequently observed on the heating equipment's fire tubes [9-10].
2.2.4 Dissolved salts effects
As the salt level of the water rises, so does the solubility of calcium carbonate. When 200,000 mg/L NaCl is added to distilled water, the solubility of CaCO3 increases from 100 mg/ to 250 mg/ When 200,000 mg/L NaCl is added to distilled water, the solubility of CaCO3 increases from 100 mg/ to 250 mg/. The higher the total dissolved solids (excluding calcium and carbonate ions), the higher the solubility of CaCO3 in water, and the lower the scaling tendency, up to a maximum of roughly 200,000 mg/L [11].
2.3 Calcium sulfate scale
The reaction that causes calcium sulfate to precipitate from water is shown in Equation 6:
![]()
Gypsum is the most common calcium sulfate deposit found in oilfields. At temperatures of less than 100 F, CaSO4.2H2O is the most common form.
2.3.1 Temperature effect
The solubility of gypsum increases with temperature until it reaches around 100 F, after which it declines. The temperature-solubility behavior of CaCO3 in the normal temperature range of interest is significantly different. Because anhydrite becomes less soluble than gypsum at temperatures above 100 F, it's reasonable to assume that anhydride would be the favored form of CaSO4 in more profound, hotter wells. Pressure and dissolved salt concentration affect the scale's temperature from gypsum to anhydrite [12].
2.3.2 Dissolved salts effect
The solubility of gypsum or anhydrite is increased by the presence of NaCl or dissolved salts other than calcium or sulfate ions, just as it is for CaCO3, up to a salt concentration of roughly 150,000 mg/L. CaSO4 solubility decreases as the salt content rises. The solubility of gypsum is tripled when 150,000 mg/L salt is added to distilled water [13].
2.3.3 Pressure effect
As we know calcium sulfate deposition can be caused by a pressure drop. Calcium sulfate becomes less soluble as pressure drop are significantly different from the reason calcium carbonate becomes less soluble. Calcium sulfate has little to do with the presence or absence of CO2 in a solution. Increased pressure has a physical effect on calcium sulfate solubility, resulting in a decrease in the size of the calcium sulfate molecule. However, massive pressure increases are required to cause a significant change in molecule size. The solubility of anhydrite in distilled water, for example, is around 0.075 wt % at 100 °C and one atmospheric pressure (14.7 psi). The solubility increases to roughly 0.09 wt % when the pressure is increased to 100 atmospheres (1470 psi) [14].
2.4 Barium sulfate scale
The scale's least soluble is barium sulfate compared to calcium carbonate and calcium sulfate is depicted in Equation 7.

If both Ba2+ and SO42- ions are present in water, scaling is likely due to BaSO4 strong insolubility. Strontium sulfate is found in most barium sulfate scales [6].
2.4.1 Temperature effect
The solubility of barium sulfate increases as the temperature rises. The solubility increases in distilled water from 2.3 mg/L at 77 F to 3.9 mg/L at 203 F. Even though the rise is significant in percentage, barium sulfate remains insoluble at this higher temperature.
If barium sulfate is non-scaling at surface conditions, it usually offers no downhole scaling concerns in an injection well due to the rise in solubility with temperature. It is more common in wells that produce or source water [15-16].
2.4.2 Dissolved salts effect
Foreign dissolved salts improve the solubility of barium sulfate in water, much as they do with calcium carbonate and calcium sulfate. At 77 F, adding 100,000 mg/L NaCl to distilled water increases BaSO4 solubility from 2.3 mg/L to 30.0 mg/L. BaSO4 solubility is increased to around 65 mg/L by maintaining 100,000 mg/L NaCl and increasing the temperature to 203 F.
Regardless of the dissolved salt content, you may predict that BaSO4 solubility will double when the temperature rises from 77 F to 203 F. The action of dissolved salts is substantially more vital, as seen by the 13-fold increase in concentration caused by the addition of 100,000 mg/L NaCl at room temperature [17].
2.5 Iron compounds scale
The presence of iron ions in water can be due to either natural occurrences or corrosion. Formation waters typically contain only a few mg/L of natural iron, with extremely rare values as high as 100 mg/L. Corrosion is always the cause of higher iron concentration. Precipitated iron compounds are efficient formation plungers and indicators of the equally significant corrosion issue [18].
2.5.1 Dissolved gases effect
CO2, H2S, or oxygen dissolved in water are the most common corrosion causes. The majority of iron-containing scales are corrosion products. Even if corrosion is minor, iron compounds can occur due to reactivity with natural formation iron. When carbon dioxide reacts with iron, iron carbonate scale forms. The pH of the system will determine whether or not scale forms. Above pH 7, scale development is substantially more likely. As a corrosion product, hydrogen sulfide produces iron sulfide, which is insoluble and forms a thin, adherent scale. "Black water" is caused by suspended iron sulfide.
Oxygen: Ferrous hydroxide, Fe (OH)2, ferric hydroxide Fe(OH)3, and ferric oxide, Fe2O3, are common scales resulting from contact with air (For example Equation 8).
![]()
When ferrous iron (Fe2+) is exposed to oxygen, it oxidizes to Fe3+, and ferric hydroxide. Above pH 4, this is almost insoluble. If oxygen was removed from the solution at pH 8, 100 ppm Fe(OH)2 (ferrous hydroxide) would still be present. The presence of suspended Fe2O3, particles, a byproduct of oxygen and iron, results in "red water." Certain bacteria (gallionella ferruginea) that thrive in water in the presence of air can also produce iron compounds. Fe++ ions from the water are taken up by these bacteria, depositing ferric hydroxide. The presence of suspended Fe2O3 particles, a byproduct of oxygen and iron, results in "red water." Certain bacteria (gallionella ferruginea) that thrive in water in the presence of air can also produce iron compounds. Fe2+ ions from the water are taken up by these bacteria, which then deposit ferric hydroxide [19].
- Preventing scale formation
3.1 Non chemical methods
3.1.1 Engineering
Due to engineering strategy, the choice of metal type for constructing plants and the selection of acceptable settings for building corrosion rate can be minimized. Recently, corrosion engineering has been attempting to create transparent plants that are more resistant to corrosion assault [1-2].
3.1.2 Cathodic protection (impressed current)
The concepts of cathodic protection are illustrated by an external power source that transforms conventional a.c. (alternating current) power to direct current (d.c.). Impressed current cathodic protection effectively stops or prevents steel corrosion (Figure 2) [22].
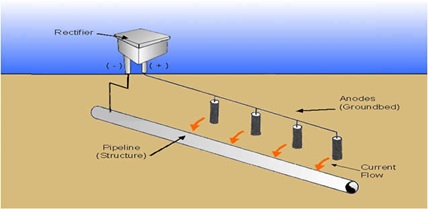
Figure 2. Cathodic protection (impressed current)
3.1.3 Sacrificial anodes
The mechanism of sacrificial anodes to protect and prevent another metal from being corroded is sacrificial anodes with high electrochemical activity compared to the protected metal, which has a lower electrochemical activity. Typically, sacrificial anodes are made of zinc alloy, magnesium alloy, or aluminum alloys. It is a simple method and inexpensive, however, the sacrificial anodes must be replaced after a certain time (Figure 3) [1].

Figure 3. (a) Sacrificial anodes on heating elements in boiler (b) Sacrificial anodes protected the pipe
3.1.4 Avoid mixing incompatible waters
The significance of avoiding compatibility issues should be clear from the preceding discussion. When it comes to combining waterways, you should always proceed with caution [23].
3.1.5 Alter water composition (water dilution)
The preceding issue is the polar opposite of this one. A scale-forming injection of water can be diluted with another water to stabilize the resulting mixture under system conditions [24].
3.1.6 Corrosion allowance
Corrosion allowance is a thickness that can be added to the outside metal surfaces to extend the lifetime of the metal. This is based on technical design (Figure 4) [25].

Figure 4. Corrosion allowance on pipe
3.2. Chemical methods
3.2.1 Scale inhibitor
Scale inhibitors are substances that, when applied to scaling water, suppress scale formation. This is performed by various mechanisms that retain the scale-forming cation (calcium, iron, and barium) "in solution." Scale inhibitors function chemically as a thin layer that coats the metals continually. On the other hand, scale inhibitors cannot be utilized in drinking water systems since the chemical nature of the inhibitor is extremely harmful [26].
3.2.2 Coatings
Coating is the oldest method of corrosion protection known to man. The principle of applying coatings for corrosion protection is to coat the metal layer with a chemical or electrochemical coat. The temperature, pressure, and corrosive agent all play a role in selecting the suitable coating. Coating, on the other hand, cannot be utilized in drinking water systems due to its highly harmful chemical nature [27-28].
3.2.3 pH Control
The solubility of iron compounds and carbonate scales increases when the pH is reduced. However, it will make the water more corrosive, perhaps causing corrosion issues. The solubility of the sulfate scale is unaffected by pH. This is not a widely utilized scale control approach. It is only useful if a slight pH change is required to prevent the precipitation of insoluble substances. pH control must also be precise, which is problematic in routine oilfield operations [2].
- Scale removal methods
4.1. Removal of scale forming constituents
4.1.1 Dissolved gas removal
Chemical and/or mechanical methods can remove dissolved gases such as H2S, CO2, and O2 from water. Insoluble iron complexes can be avoided due to this (sulfide, oxides).
Removing CO2 from the water will make scale deposition more severe. However, the pH can be decreased to where all carbonates and bicarbonates are converted to CO2. The CO2 will then be removed, preventing the production of carbonate scales. One of the principal causes of corrosion is the presence of one or more of these three dissolved gases in water [29].
4.1.2 Water softening processes
Scale deposition from injection waters is rarely prevented using processes such as ion exchange, precipitation softening, or distillation. These methods can be employed alone or in combination to remove scale-forming ions such Ca++, Mg++, SO4 - -, HCO3 -. By removing these ions, they "soften" the water. Applying these procedures with oilfield brines is the cost of removing the necessarily massive amounts involved. Using other scale control methods is almost always much less expensive. Ion-exchange is perhaps the most often used of the procedures listed in oilfield operations. Ion exchange units have long been utilized to soften fresh water as boiler feed water for thermal recovery stream generators. They are also widely used in numerous gas processing plants for the same purpose [30].
4.2 Scale removal by chemicals
4.2.1 Hydrocarbons
Hydrocarbons, while not technically a scale, are frequently present and can significantly obstruct the action of acid or other scale-removal agents. Acid will not react with a scale that has been coated in oil. Because a hydrocarbon solvent is required to remove any oil, paraffin, or asphaltic components from the scale, the choice of a hydrocarbon solvent is usually made by trial. Carbon tetrachloride is a tremendous all-around solvent, but it's dangerous and difficult to dispose of. Because of its low flash point, carbon disulfide is too risky to use [31-32].
4.2.2 Calcium carbonate
Under most circumstances, HCl is recommended as the cheapest and most straight forward technique to dissolve CaCO3 scale. HCl concentrations of 5, 10, or 15% are commonly used (Equation 9).
![]()
A corrosion inhibitor must be put to it to prevent the acid from dissolving the pipe. A surfactant is frequently added to help remove any oil layer off the scale a pre-wash with a solvent is preferred unless the amount of oil is extremely smalminimal. a pre-wash with a solvent is preferred. Oily scale will not react with acid [33].
EDTA (Ethylenediaminetetraacetic acid) is a substance that "chelates" or forms a soluble complex with the calcium ion. It is, however, usually too costly to utilize for routine oilfield cleanout operations. 7.4 ppm EDTA is required to chelate 1.0 ppm Ca++ [34].
4.2.3 Calcium sulfate (Gypsum)
Calcium sulfate is unaffected by hydrochloric acid.
Carbonates or hydroxides react with calcium sulfate and "transform" it to acid-soluble calcium carbonate or calcium hydroxide. After the conversion treatment, the calcium carbonate or calcium hydroxide is dissolved in hydrochloric acid.
Ammonium carbonate, which is sold under various trade names, is a excellent example of this sort of chemical. The reaction is depicted as Equation 10:
![]()
After that, the calcium carbonate is dissolved in HCl (Equation 11):
![]()
The CO2 produced by the acid reaction aids in the mechanical removal of any remaining deposit, moreover used are organic converters such sodium citrate, potassium glycollate, and potassium acetate, these compounds react with calcium sulfate deposits causing them to sell and soften, allowing them to be flushed away easily. These chemicals are costly, need several hours of contact time to work on thick deposits, and should be tested on a absolute scale sample before use, if possible [35-36]. At the same time, when compared to acidic solutions, calcium sulfate, EDTA has a higher solubility in EDTA solution. On the hand, 10% NaOH solution can dissolve up to 12.5 percent of the weight of the gypsum scale [34]. At 100 Fahrenheit degrees, water containing 55,000 mg/L NaCl dissolves three times as much gypsum as fresh water [37].
4.2.4 Iron compounds
Iron compounds are usually dissolved using hydrochloric acid. To avoid pipe corrosion, it must once again contain a corrosion inhibitor. Iron sequestering agents are frequently used as an addition. The iron will not be able to re-precipitate if a sequestering agent is used. If the acid is depleted and the pH rises to a high enough level, this can happen. Care should be taken when dissolving iron sulfide.
The reaction between FeS and HCl is (Equation 12):
![]()
H2S is highly deadly, and even a few ppm in the air can be fatal to humans. If there is a chance that personnel will inhale H2S, fresh air masks must be on hand and in use , an another way to remove iron oxides can be removed with citric acid, but usually in the oilfield, it is not widely utilized [38].
4.2.5 Barium sulfate
Chemically, dense barium sulfate is nearly tough to remove. A little bomb would be instrumental in many situations, but it is rarely practicable. EDTA can assist, but the chances of success are slim.
4.3. Scale removal from surface lines
Chemicals and line scrapers, often known as "pigs," are commonly used to remove scale from surface lines. Pigs with a malleable plastic foam body covered with a rough or abrasive material are the most popular and effective (Figure 5). These pigs may be pumped through a sequence of different-sized lines, and their bodies will bend sufficiently (within limits) to allow them to scrape effectively [39].
The following processes might be included in a normal flow line cleaning to remove oil coated calcium carbonate from a line. A slug of solvent follows a pig. A slug of HCl follows a pig. Use a neutralizing solution (high pH water) or a thorough water wash to eliminate all acid. Acid inhibitors degrade with time, and all acid, spent or not, must be flushed out of the line to avoid severe pitting corrosion.

Figure 5. The pig movement into pipe cleaning deposits scale
A solvent-acid emulsion is an excellent choice if the carbonate scale is layered, with alternate layers of scale and oil. Pigs should be used again since the mechanical scraping motion is highly useful in boosting the effectiveness of any solvent system. The pumping rate is another essential factor to consider while cleaning out surface lines. It takes a certain length of time for any solvent to get saturated with the substance it dissolves. As a result, a particular amount of contact time with the scale surface is required to accomplish a good job. If you pump acid into one end of a line and it comes out the other end under-saturated concerning the substance to be dissolved, you may not have left it in the line long enough. Slow down your pumping. In rare cases, it may be necessary to stop pumping and let the solvent soak for a while.
- Scale corrosion monitoring methods
5.1 Scale corrosion coupons
Corrosion coupons must have their composition, weight, size, and density determined. Corrosion coupons can provide you with accurate corrosion rates in your system. It can also provide a qualitative visual indication of the type of degradation occurring in the monitored system. The two most frequent types of corrosion coupons are scale and corrosion. Corrosion coupons are the most effective tools for determining corrosion rate; strip coupons can be measured in weight mils per year, whereas scale coupons' corrosion rate is assessed by open and closed holes (Figure 6) [1, 40].

Figure 6. Shown (a) strip corrosion coupon (b) scale corrosion coupon
5.2 Electrical resistance(ER) probes
When paired with high-sensitivity electrical resistance sensors, the electrical resistance technique allowed for the detection of corrosion loss; nevertheless, salt may precipitate on probes, resulting in erroneous results [41-42].

Figure 7. Electrical resistance (ER) probes fixed into pipe
5.3 Field scale analysis
In a field analysis, we determine the solid's composition by conducting the following: (1) Soak the sample in a solution to dissolve any hydrocarbons. If the solvent darkens in color, keep an eye on it. (2) Check the sample for magnetic properties. If it is strongly magnetic, it most certainly contains a significant proportion of Fe3O4 (magnetic iron oxide). If it is weakly magnetic, it may include a small amount of Fe3O4 or by iron sulfide. (3) Fill a 15% HCl bottle with the sample. Keep track of whether or whether there is a violent reaction. Take note of any odors. (H2S denotes FeS.) Take note of the acid's hue. An iron compound is indicated if it turns yellow. (4) Check the solubility of the substance in water. NaCl dissolves in water. Table 2 summarizes the qualitative identification of the components present in terms of the previous properties.
Table 2. Summarizes the qualitative identification of the components present in the previous properties

- Conclusions
Scale corrosion is the worst type of corrosion when compared to other types of corrosion because: first, it can attack all areas of the pipes; second, it can reduce pipe diameter; third, production flow rate will decrease, resulting in production losses while pressure returns; and fourth, scale corrosion's tendency to attack pipes is higher even when the pipes are made of non-metals such as polymers. Scaling can cause technical issues such as equipment and pipe obstruction, and significant damage and financial losses.
Calcium carbonate, calcium sulfate, barium sulfate, and iron compound are the four primary scale forms. Barium sulfate scale has a lower solubility than the calcium carbonate and calcium sulfate scale. The likelihood of calcium carbonate scale formation increases with temperature, decreases as CO2 partial pressure lowers, increases as pH rises, and decreases as total dissolved salts fall. Calcium sulfate solubility was affected by temperature, and calcium sulfate became less soluble as the pressure decreased. The solubility of barium sulfate increases as the temperature rises, and this is based on the fact that the solubility of barium sulfate rises as well. Two methods to preventing scale formation non-chemical methods and chemical methods; non-chemical methods have the advantages of being simple, inexpensive, and simple to use; however, there are drawbacks such as higher current on cathodic protection systems potentially maybe damaging the pipe, and sacrificial anodes needing to be replaced after a period of time. Chemical techniques have the advantage of being able to cover all areas of metals. However, they have some drawbacks, such as being inexpensive and requiring a trial to find the right chemical. The best treatment for salt buildup is a fresh water wash. The best chemicals for removing scale are HCl and EDTA, whereas citric acid can be used to remove iron oxides. However, HCL is not appropriate for removing the calcium sulfate scale, whereas sodium hydroxide is used. Pig launchers, on the other hand, are a non-chemical way of removing scale deposits; nevertheless, they may scrape the pipe and are not ideal for use inside pipes with smaller radii where pressure is required for movement. Sand, silt, and clay are commonly found in scale deposits as occluded particles. These materials can be rinsed out once the bulk scale material has been dissolved. Scale coupons and a lab analysis method are critical for monitoring and controlling scale inclination. Scale coupons are the most excellent tools for monitoring corrosion in the absence of ERP sensors. The downside with ERP devices is that even a tiny quantity of salt put on the electrode can cause incorrect results, whereas scale coupons require operator skills.


